2. 犹他大学 材料科学与工程系, 美国 犹他州 盐湖城 84112
2. Department of Material Science and Engineering, University of Utah, Salt Lake City, UT 84112 USA
Nowadays a lot of attention is being paid on the development of methods and instrumentation for the detection of explosives and illicit chemicals, because of the threat of terrorist use of explosive device and chemical, biological or radioactive agents[1,2,3,4,5]. Additionally, the analysis of explosives is also of great importance for environmental cleaning, forensic science and military operations[6]. Many techniques have been investigated for the detection of explosives, such as gas chromatography coupled with mass spectroscopy, nuclear quadrupole resonance, energy dispersive X-ray diffraction, neutron activation analysis, electron capture detection, ion mobility spectrometry, and cyclic voltammetry[7, 8]. Most of them, though highly sensitive, are expensive and require sophisticated instrumentation that is not easily applied to on site field testing. For the large area searching or personal control, sniffer dogs are the most successful explosives detection systems at present. On the other hand this method is not well-suited for continuous monitoring because dogs are living beings and show behavioral variations. So improvements of existing sensor platforms along with development of new chemical sensors are always needed.
Explosive compounds can be classified according to their chemical structures[5, 7]. The chemical structures of some common explosives are shown in Figure 1. These include the nitroaromatics such as trinitrotoluene (TNT), picric acid (PA), and 2,4,6-trinitrophenylmethylnitramine (Tetryl); and nitroalkanes such as nitromethane and 2,3-dimethyl-2,3-dinitrobutane (DMNB, tagging/detecting agent for plastic bonded explosives); and nitramines such as 1,3,5-trinitro-1,3,5-triazacyclohexane (RDX), octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX); and nitrate esters such as pentaerythritol tetranitrate (PETN); and peroxides such as TATP. One property of the nitro explosives which may be exploited in detection schemes is their electron accepting capability[4]. The electron deficient molecules are able to form complexes with electron rich fluorophores, which has been widely used to detection them via electron-transfer-induced fluorescence quenching. While, peroxide-based explosives have a strong oxidant character, which has been used to design fluorogenic and chromogenic probes based on redox reactions coupled by color/emission changes[5,9,10].
 | Figure 1 Chemical structures of common explosives |
Substitution of the electron-withdrawing nitro groups make the explosive molecules highly electron-deficient and readily to accept electrons from excited fluorophores. Electron-transfer-induced fluorescence quenching is the most practical and efficient mechanism of signal transduction for the detection of explosives. A basic frontier molecular orbital based mechanism for electron transfer fluorescence quenching is illustrated in Figure 2[2]. The highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) of the electron donor (fluorophores) and acceptor (nitro explosives) are illustrated in this simple diagram. In the ground state of donor and acceptors, electron transfer reaction cannot be happened because there is no energetic driving force. When a fluorophore is excited by UV/Vis radiation, an electron is promoted into a higher energy states (LUMO). In the absence of an acceptor, a photon is emitted when the fluorophore relaxed back to the ground state, resulting in the emission of light. Otherwise, because of the LUMO of acceptor is lower in energy than the LUMO of donor, there is a significant energetic driving force to lower the energy of the system via an electron transfer from donor to acceptor, resulting in oxidation of donor and concomitant reduction of acceptor, giving an ion radical pair. Because the singlet excited state of donor has been transformed in this reaction, it can no longer emit, and typically the fastest process is simple reverse electron transfer from the LUMO of acceptor to singly occupied HOMO of donor, which is non-radiative in nature.
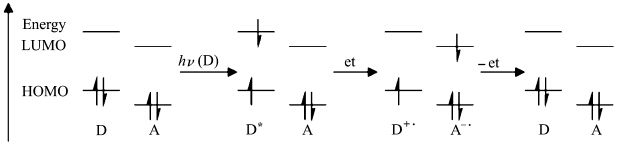 |
Figure 2 A molecular orbital diagram for photoinduced charge transfer (“et” refers to electron transfer; “-et” refers to reverse electron transfer)[2] |
In principal two major approaches to detect explosives can be distinguished: bulk detection and trace detection[11]. Bulk detection of explosives is very common, e.g., in airport security. Usually, explosive material is detected directly by viewing images made by x-ray scanners or similar equipment. In trace detection, the explosive is detected by chemical identification of microscopic residues of the explosive compound. These residues can be in either or both of two forms: vapor and particulate. Vapor refers to the gas-phase molecules that are emitted from a solid or liquid explosive because of its finite vapor pressure. Particulate contamination refers to microscopic particles of solid material that adhere to surfaces that have, directly or indirectly, come into contact with an explosive material. Trace detection of explosives is not easy. One reason is the low vapor pressure of most explosives. Figure 3 shows a few commonly used high explosives and their vapor concentration in saturated air[11, 12]. Some of the explosive, such as EGDN and DNT, having saturated vapor concentrations in air close to or greater than one part per million (1 ppm), can be seen as volatile or high vapor pressure explosives. The low-vapor-pressure explosives, such as HMX, RDX and PETN, have saturated vapor concentrations in air near or below the one part per trillion (1 ppt). They can be seen as non volatile particles and are almost impossible detected in vapor phase. TNT, one of the more ubiquitous explosives used, has a moderate vapor pressure (approximately 5 ppb at room temperature) and is possible to be detected both in vapor phase and in solid.
 | Figure 3 Vapor concentrations of high explosives in saturated air at 25 ℃[12] |
Chemical sensors can be broadly classified as optical sensor or electronic sensor based on optical or electronic readout mechanisms. Due to the importance and widespread interests of explosive sensing, several review articles with emphasis on different aspects have been published[2-5,13-16].This review will focus on research works of Prof. Ling Zang′s group and Prof. William Trogler′s group at University of Utah and University of California, San Diego, respectively. We will briefly introduce the optical sensor via fluorescence quenching based on one-dimensional small molecular nanomaterials, fluorescent coordination polymers and polysiloles. Then we will demonstrate the researches on electronic sensors based on semiconductor phthalocyanine thin films or organic photoconductive nanowires. 2 One-dimensional small molecular nanomaterials
While the detection of explosives in the vapor phase can be a difficult task, it must be said that there are some very good, reliable sensors that operate by vapor phase detection. The pentiptycene based conjugated polymer sensor developed by the Swager group is an example of a very sensitive vapor phase sensor (Figure 4)[15,16,17,18]. A conjugated polymer can be seen as a “molecular wire” because each repeat unit in the polymer chain is electronically coupled to the adjacent unit. And exciton has the ability to travel throughout the conjugated polymer backbone. If the repeat units are analyte receptors, this mechanism of exciton transport can result in a large degree of amplification, because with one binding an entire polymer chain is quenched. It like light bulbs wires in series, in which the extinguishing of one bulb turns off the entire network of lights. Pentiptycene moiety is a relatively large and very rigid three-dimensional side group that prevents aggregation and self-quenching, yet still allows efficient exciton transport. However, the quenching efficiency of these materials is often limited by the short exciton diffusion because of the poor molecular organization and/or weak intermolecular electronic interactions. To achieve desirable amplification of signal transduction, very thin films are needed. While, sufficiently thick film is usually required to produce measurable fluorescence intensity and to minimize the interference of photobleaching.
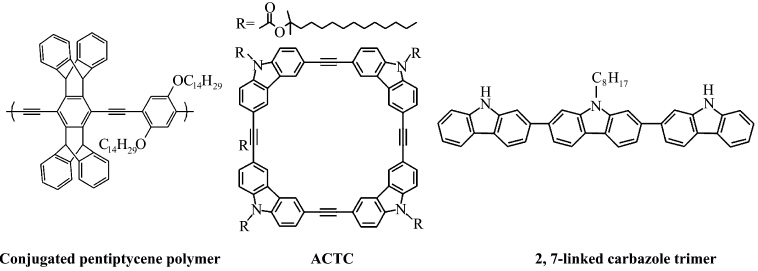 | Figure 4 Molecular structures of conjugated pentiptycene polymer, ACTC and 2,7-linked carbazole trimer |
Zang′s group found that planar aromatic molecules can self-assembly into one-dimensional nanostrctures that exhibit uniaxial optical properties along the π-π stacking direction[19,20,21]. And the long-range molecular arrangement also leads to 1D enhanced exciton migration (via intermolecular π-electronic coupling) along the long axis of the nanofiber, enabling amplified fluorescence quenching by the surface adsorbed quencher molecules. They firstly reported that carbazole-cornered, arylene-ethylene tetracycle (ACTC) formed a porous, nanofibril film with strong fluorescence by surface casting (Figure 4)[20]. The incorporation of carbazole enhanced the electron donating power of the molecular and thus increased the efficiency of fluorescence quenching by oxidative explosives. These films exhibit efficient fluorescence quenching upon exposure to the saturated vapor of DNT and TNT (Figure 5A). And the quenching shows little dependent to the film thickness and the fluorescence can be slowly recovered by exposure to air or quickly recovered by exposure to the vapor of hydrazine (Figure 5B).
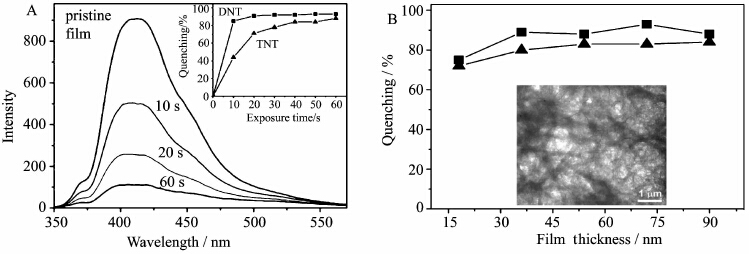 |
Figure 5 (A) The fluorescence of ACTC film was quenched by a saturated vapor of TNT (5 ppb) at various time
The inset showed the time-course of quenching for TNT and DNT (B) Thickness dependence of fluorescence quenching efficiency of ACTC films The quenching was tested upon exposing the film to saturated vapor of TNT (▲) and DNT (■) for 60 s The inset is a TEM image of a thin film of ACTC cast on silicon oxide from 2 mmol/L THF solution [20] |
However, both pentiptycene conjugated polymers and ACTC impose much difficulty to synthesis because they require multistep synthetic process and/or special catalysts for making the precursors. Recently, Zang′s group reported 2,7-linked carbazole trimer can be synthesized in one-step by Suzuki-coupling reaction (Figure 4)[22]. The nanofibril film of carbazole trimer demonstrated the same efficient, fast fluorescent sensing for nitroaromatic explosives (e.g., TNT, DNT) as ACTC film. Furthermore, the sensor also showed good response to nitroaliphatic explosives (e.g., nitromethane), which remain difficult to detect due to their high volatility and low chemical binding to many sensory materials, and implied the potential applications for on-site explosive monitoring. 3 Fluorescent coordination polymers
Coordination polymers, or metal-organic frameworks (MOFs), constructed from metal ion connectors and polydentate bridging ligands, have drawn growing interest in chemistry and materials science due to their unique and highly tailorable properties and potential applications in heterogeneous catalysis, drug delivery, imaging, and sensing[23,24,25].Fluorescent MOFs have good potential to sense explosives because of porosity, high surface area, which may allow more analyte molecules to come into contact with the MOF surface and may reach high sensitivity[26, 27].
Prof. Jing Li′s group reported that highly luminescent microporous crystalline metal-organic framework (MMOF) [Zn2(bpdc)2(bpee)], can be able to highly sensitively detect nitroaromatic explosive DNT and plastic explosive taggant DMNB[26]. The outstanding sensing capability may be attributed to the infinite 3D framework structure and inherent microporosity. The network structure of MMOF, containing π moieties, may also facilitate the excitons migration like conjugated polymers. Their latest work demonstrated a possible way to selectively detect explosives of different types(eg, aromatic DNT vs aliphatic DMNB)[28].
Scaling down these materials lead to highly tailorable nanomaterials know as nanoscale coordination polymers (NCPs)[30,31,32]. Zang′s group reported a nanoscale zinc(II)-carboxylate coordination polymers with a π-conjugated ligand (Zn-BCPA)[29]. The nanomaterial has a cubic morphology with strong fluorescence and efficient emission quenching upon interaction with the nitro-based explosives including DNT, TNT and the high volatile nitromethane (Figure 6). For example, when Zn-BCPA NCP film was blew over by nitromethane vapor with a concentration as low as 360 ppm (1% of saturated nitromethane vapor in air), its emission intensity was quenched by 10%, monitored by a portable spectrometer, which make them promising sensory materials for infield explosives monitoring.
 |
Figure 6 (A) Molecular structure of Zn-BCPA coordination polymer (B) SEM image of Zn-BCPA nanocubes (Insets are the photoimages of Zn-BCPA NCPs under daylight and UV illumination at 354 nm respectively) (C) Emission intensity as monitored at 450 nm for a film of Zn-BCPA NCPs upon blowing over with 360 ppm nitromethane vapor[29] |
Silole is one kind of metallacylopenta-2,4-dienes, or metalloles, which are analogous to cyclopentadiene but have a Group 14 (IVA) element (Si, Ge, Sn, Pb) substituted at the sp3 carbon[4]. As seen in Figure 7, low lying σ* orbitals of the silicon atom have a favorable overlap of that of the π* orbitals of cyclopentadiene organic framework. This favorable interaction produces fluorescence with relatively low energy excitation states. Polysiloles possess the same features including a low reduction potential, low lying LUMO and visible fluorescence. These make the polysiloles ideal sensing materials via fluorescence quenching with amplification effect due to electron delocalization in these polysiloles.
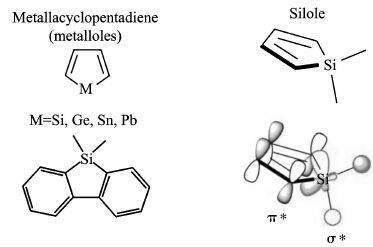 | Figure 7 Molecular structures of metalloles, silole and silafluroene[33] |
Trogler′s group has deeply researched the synthesis of polysiloles and their sensing applications[9,33,34,35,36,37,38]. Detection of nitroaromatic explosives, such as DNT, TNT and PA, was explored in organic or aqueous solution. Besides that, one of the most interesting works was fluorescence sensing for explosive particulate. As we have described above, some of common high explosives, such as, PETN, RDX and HMX, have very low vapor pressure (near or below 1 ppt) at room temperature. Considered the environmental surfaces will also trap vapor molecules, the amount in the vapor phase will even lower. These promoted researcher to look upon particulate as target analytes. Whatever in solution or vapor phase, the sensing system requires the analyte to have effective interaction with sensor. The advantage of their detection as particulate or in the solid state is that the affinity for the sensor can be eliminated.
Sohn, et al first demonstrated surface detection method for the analysis of solid particulates of TNT[38]. A polysilole thin layer was coated by spray-coating onto filter paper or other substrates which were contaminated with TNT. Then the substrate can be illuminated with a small ultraviolet (UV) lamp, while TNT residue will quench luminescence on the substrate. The quenched area could be easily distinguished from the region unaffected by naked eyes, which make trace residue of TNT visualize. The explosives studied by this detection method were expanded to include PA, RDX, HMX, Tetryl, TNG, and PETN[33,34,39].And the detection limits are reported in nanograms per square centimeter to emphasize the quantity of explosive deposited in the given area. Some examples of this mehod can be seen in Figure.8, where various amount of RDX were deposited onto filter paper, tetryl was deposited in ceramic wells, and PETN successive thumbprints were all visible after a polysilole solution was applied. The improved method has negated the vapor pressure issue and allowed for the detection of high explosives that were previously not attainable.
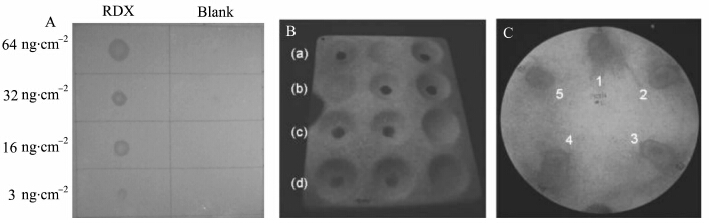 |
Figure 8 Examples of fluorescence quenching of thin films of silafluorene polymers by solid particulates of
various explosives imaged with a Sony 2.0 megapixel digital camera under continuous UV excitation (302 nm)
(A) Detection of RDX particulates on filter paper (B) Detection of tetryl particulates (a) 64, (b) 32, (c) 16, and (d) 3 ng·cm-2 on a porcelain tray. The analyte was randomly place in 2 of 3 wells, and observed quenching was confirmed by an independent observer (C) Detection of five successive thumbprints on filter paper contaminated with production-line PETN particulates[33, 34] |
Sensors that convert a chemospecific sensing event into a voltage or current output are broadly classified as electronic sensors[40]. The advantages of electronic sensors are their low power requirements, sensitivity, and easy integration for sensor arrays, while the issues such as selectivity and stability baffled their practical applications. The electronic sensor types would include resistive, capacitive, surface acoustic wave (SAW), electrochemical, and mass (e.g., quartz crystal microbalance (QCM) and microelectromechanical systems (MEMSs)). The sensitivity and selectivity of sensors rely on the materials designed, which will change their electronic properties in specific ways when in contact with volatile compounds.
Although photoluminescent sensors have been seen as most efficiency for explosive sensing as we described above, improvised peroxide explosives cannot be detected in this way. Recent incidents in England and Germany involving peroxide based explosives have made peroxide detection crucial to counterterrorism efforts. Vapor phase monitoring of peroxides has been a topic of critical importance. As reported by Trogler′s group, chemiresistors based on thin films of methal phthalocyanies (MPcs, M=Co, Ni, Cu, and H2) are examined as sensors for vapor phase H2O2 detection under practical conditions (i.e., in the presence of ambient humidity) (Figure 9A)[41]. Selectivity is gained from the ability of H2O2 to cause current losses in CoPc sensors and current gains in NiPc, CuPc, and H2Pc sensors (Figure 9B,C). The reason for large current losses is that cobalt-catalyzed oxidation of H2O2 on the surface of CoPc is occurring (with concurrent film reduction), leading to hole trapping and loss of current. In contrast, MPcs with nonredox-active metal centers (M=Ni, Cu, and H2) were revealed no electrocatalytic behavior in the presence of H2O2. However, small amount of hydroxyl radicals were generated, leading to the observed sensor response as radical reaction or as charge-transfer processes.
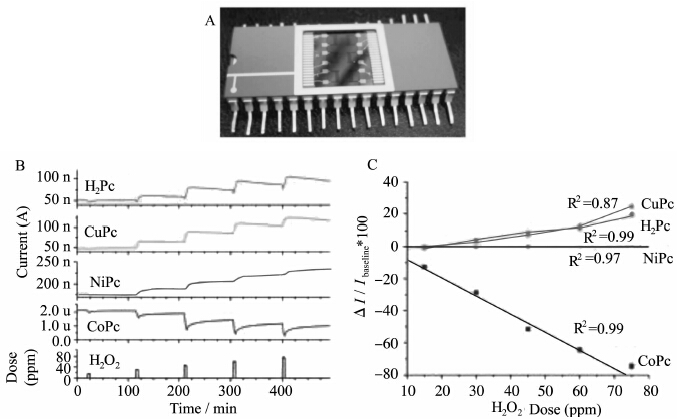 |
Figure 9 (A) MPc sensor array containing six chemiresistors wirebonded in a dual-inline ceramic package (B) Sensing data for MPcs (M=Co,Ni,Cu,H2) exposed to varied concentration doses of 27% H2O2(aq) (C) Quantitative sensor response data plotted for each dose in (B)[41] |
This is the first example of contrasting analyte redox behavior dependent on the MPc metal center in a chemiresistor. Due to its high catalytic activity, CoPc is the most sensitive material for detecting H2O2, with a detection limit of 50 ppb, much better than that of commercial H2O2 sensors (in the range of 0.1 to 1 ppm). And the research on ChemFETs based on vapor deposited or solution-processed phthalocyanine thin-films is ongoing to detect organic peroxide (such as TATP).
Zang′s group also reported an electronic sensor based on organic nanoribbons for nitro explosive sensing[42]. The nanoribbons were self-assembly from soluble perylene diimide (electron acceptor) with various donor groups (Figure 10). Their photoconductivity is much higher than dark current because the good kinetic balance between the intramolecular charge recombination (between D and A) and the intermolecular charge transport along the nanoribbon. The photogenerated electrons can be efficient trapped by oxygen molecules or other oxidizing molecules (such as nitro compounds), leading to depletion of the charge carriers or current loss. Having these findings, the authors constructed the electrical vapor sensors that relies on the photocurrent modulation and takes advantage of the enhanced gas adsorption intrinsic to the porous nanofibril film, and proved to be effective sensing nitro-based explosives, including nitromethane, nitrobenzene, 4-nitrotoluene, and 1-chloro-4-nitrobenzene. Furthermore, the sensors have negligible current response to many other common solvents and some reductive reagents such as aniline. That demonstrated the good sensing selectivity.
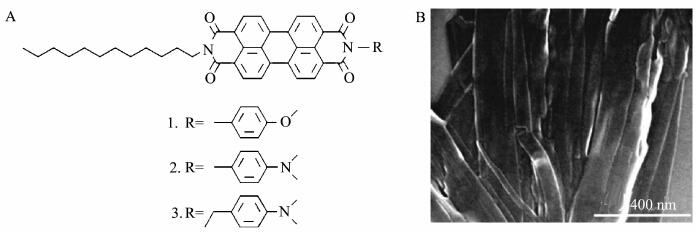 |
Figure 10 (A) Molecular structures of perylene diimide with various electron donor groups (B) SEM images of nanoribbons fabricated from molecular 3[42] |
6 Conclusions and future perspectives
Besides Zang′s group and Trogler′s group, a few groups are active in the explosive sensing research, which promoted the development of commercial products[43,44,45,46]. Due to the advantages of fluorescence quenching method and the signal amplifying effect of conjugated molecules, portable trace explosive sensors based on polysiloles and small molecular nanofibrils have been fabricated with the commercial name “RedX Defence XPAK” and “Vaporsens”, respectively[47, 48]. As most fluorescence quenching sensors are limited to nitroaromatic-based explosives, trace detection of other classed (e.g., nitramine, nitrate, and peroxide) with low vapor pressure is still a challenge. And optical sensor based on "turn-on" mechanism remains an area in need of further progress. Very recently, sensors based on luminescent MOFs have attracted a great deal of attentions because the luminescent properties are highly sensitive and potentially selective to guest species.
Electronic sensors are valued for their low power requirements, sensitivity, and potential for mass fabrication in a compact form. The technology for measuring voltage and current is highly sensitive and of low cost, which makes such sensors attractive as compact inexpensive sensors. However, issues such as chemical sensitivity, selectivity, and stability pose challenging practical barriers to their application.
Acknowledgement
This work was supported by NSF (CAREER CHE 0641353, CBET 730667), DHS (2009-ST-108-LR0005), and the USTAR Program.
| [1] | Krausa M, Reznev A A. Vapour and Trace Detection of Explosives for Anti-Terrorism Purposes[M]. Boston: Kluwer Academic Publishers,2003. |
| [2] | Marshall M,Oxley J C. Aspects of Explosives Detection[M]. Boston: Elsevier,2009. |
| [3] | Germain M E, Knapp M J. Optical explosives detection: from color changes to fluorescence turn-on[J]. Chem. Soc. Rev., 2009, 38: 2543-2555. |
| [4] | Toal S J, Trogler W C. Polymer sensors for nitroaromatic explosives detection[J]. J. Mater. Chem., 2006, 16: 2871-2883. |
| [5] | Salinas Y, Martinez-Manez R, Marcos M D, Sancenon F, Costero A M, Parra M, Gil S. Optical chemosensors and reagents to detect explosives[J]. Chem. Soc. Rev., 2012, 41: 1261-1296. |
| [6] | Yinon J. Forensic and Environmental Detection of Explosives[M]. New York: Wiley, 1999. |
| [7] | Yinon J,Zitrin S. Modern Methods and Applications in Analysis of Explosives[M]. New York: John Wiley,1996. |
| [8] | Moore D S. Instrumentation for trace detection of high explosives[J]. Rev. Sci. Instrum., 2004, 75:2499-2512. |
| [9] | Sanchez J C, Trogler W C. Polymerization of a boronate-functionalized fluorophore by double transesterification: applications to fluorescence detection of hydrogen peroxide vapor[J]. J. Mater. Chem., 2008, 18:5134-5141. |
| [10] | Xu M, Bunes B R, Zang L. Paper-based vapor detection of hydrogen peroxide: colorimetric sensing with tunable interface[J]. ACS Applied Materials & Interfaces, 2011, 3:642-647. |
| [11] | Rhykerd C L, Hannum D W, Murray D W,Parmeter J E.Guide for the Selection of Commerial Explosives Detection Systems for Law Enforcement Applications[M]. Sandia National Laboratories, 1999. |
| [12] | Martinez H P. Luminescent Organosilicon Polymers and Sol-Gel Synthesis of Nano-Structured Silica[M]. San Diego: University of California, 2011. |
| [13] | Caygill J S, Davis F,Higson S P J. Current trends in explosive detection techniques[J]. Talanta, 2012, 88: 14-29. |
| [14] | Senesac L,Thundat T G. Nanosensors for trace explosive detection[J]. Mater. Today, 2008, 11: 28-36. |
| [15] | Thomas S W,Joly G D,Swager T M. Chemical sensors based on amplifying fluorescent conjugated polymers[J]. Chem. Rev., 2007, 107:1339-1386. |
| [16] | McQuade D T, Pullen A E, Swager T M.Conjugated polymer-based chemical sensors[J]. Chem. Rev., 2000, 100: 2537-2574. |
| [17] | Yang J S, Swager T M. Porous shape persistent fluorescent polymer films:an approach to TNT sensory materials[J]. J. Am. Chem. Soc., 1998, 120: 5321-5322. |
| [18] | Yang J S, Swager T M. Fluorescent porous polymer films as TNT chemosensors:electronic and structural effects[J]. J. Am. Chem. Soc., 1998, 120: 11864-11873. |
| [19] | Zang L, Che Y, Moore J S. One-dimensional self-assembly of planar π-conjugated molecules: adaptable building blocks for organic nanodevices[J]. Acc. Chem. Res., 2008, 41: 1596-1608. |
| [20] | Naddo T, Che Y, Zhang W, Balakrishnan K,Yang X,Yen M, Zhao J, Moore J S,Zang L. Detection of explosives with a fluorescent nanofibril film[J]. J. Am. Chem. Soc., 2007, 129: 6978-6979. |
| [21] | Che Y, Yang X,Loser S, Zang L. Expedient vapor probing of organic amines using fluorescent nanofibers fabricated from an n-type organic semiconductor[J]. Nano Lett., 2008, 8:2219-2223. |
| [22] | Zhang C,Che Y, Yang X, Bunes B R, Zang L. Organic nanofibrils based on linear carbazole trimer for explosive sensing[J]. Chem. Commun., 2010, 46: 5560-5562. |
| [23] | Wang X, McHale R. Metal-containing polymers: building blocks for functional (nano)materials[J]. Macromol. Rapid Commun., 2010, 31: 331-350. |
| [24] | Leong W L, Vittal J J. One-dimensional coordination polymers: complexity and diversity in structures, properties, and applications[J]. Chem. Rev., 2010, in press. |
| [25] | Farrusseng D, Aguado S, Pinel C. Metal-organic frameworks: opportunities for catalysis[J]. Angew. Chem. Int. Ed., 2009, 48: 7502-7513. |
| [26] | Lan A, Li K, Wu H, Olson D H,Emge T J, Ki W,Hong M,Li J. A luminescent microporous metal-organic framework for the fast and reversible detection of high explosives[J]. Angew. Chem. Int. Ed., 2009, 48: 2334-2338. |
| [27] | Allendorf M D, Bauer C A, Bhakta R K, Houk R J T. Luminescent metal-organic frameworks[J]. Chem. Soc. Rev., 2009, 38: 1330-1352. |
| [28] | Pramanik S, Zheng C, Zhang X, Emge T J, Li J.New microporous metal-organic framework demonstrating unique selectivity for detection of high explosives and aromatic compounds[J]. J. Am. Chem. Soc., 2011, 133: 4153-4155. |
| [29] | Zhang C, Che Y, Zhang Z, Yang X, Zang L. Fluorescent nanoscale zinc(Ⅱ)-carboxylate coordination polymers for explosive sensing[J]. Chem. Commun., 2011, 47:2336-2338. |
| [30] | Zhang X, Ballem M A, Ahreén M, Suska A, Bergman P, Uvdal K. Nanoscale Ln(III)-carboxylate coordination polymers (Ln=Gd, Eu, Yb): temperature-controlled guest encapsulation and light harvesting[J]. J. Am. Chem. Soc., 2010, 132: 10391-10397. |
| [31] | Hui J K H, MacLachlan M J. Metal-containing nanofibers via coordination chemistry[J]. Coord. Chem. Rev., 2010, 254: 2363-2390. |
| [32] | Horcajada P, Chalati T, Serre C,Gillet B, Sebrie C,Baati T, Eubank J F,Heurtaux D, Clayette P, Kreuz C, Chang J S,Hwang Y K, Marsaud V, Bories P N,Cynober L, Gil S, Ferey G,Couvreur P, Gref R.Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging[J]. Nat. Mater., 2010, 9: 172-178. |
| [33] | Sanchez J C, DiPasquale A G, Rheingold A L, Trogler W C. Synthesis, luminescence properties, and explosives sensing with 1,1-tetraphenylsilole- and 1,1-silafluorene-vinylene polymers[J]. Chem. Mater., 2007, 19: 6459-6470. |
| [34] | Sanchez J C, Urbas S A, Toal S J, DiPasquale A G, Rheingold A L, Trogler W C. Catalytic hydrosilylation routes to divinylbenzene bridged silole and silafluorene polymers. applications to surface imaging of explosive particulates[J]. Macromolecules, 2008, 41: 1237-1245. |
| [35] | Toal S J, Magde D,Trogler W C. Luminescent oligo(tetraphenyl)silole nanoparticles as chemical sensors for aqueous TNT[J]. Chem. Commun., 2005, 5465-5467. |
| [36] | Toal S J, Jones K A, Magde D, Trogler W C. Luminescent silole nanoparticles as chemoselective sensors for Cr(VI)[J]. J. Am. Chem. Soc., 2005, 127: 11661-11665. |
| [37] | Sohn H, Sailor M J, Magde D, Trogler W C. Detection of nitroaromatic explosives based on photoluminescent polymers containing metalloles[J]. J. Am. Chem. Soc., 2003, 125: 3821-3830. |
| [38] | Sohn H, Calhoun R M, Sailor M J, Trogler W C. Detection of TNT and picric acid on surfaces and in seawater by using photoluminescent polysiloles[J]. Angew. Chem., 2001, 113: 2162-2163. |
| [39] | Sanchez J C, Trogler W. C. Efficient blue-emitting silafluorene-fluorene-conjugated copolymers: selective turn-off/turn-on detection of explosives[J]. J. Mater. Chem., 2008, 18: 3143-3156. |
| [40] | Trogler W.Chemical Sensing with Semiconducting Metal Phthalocyanines[M]. Mingos D M P,Day P, Dahl J P, Eds. Springer Berlin/Heidelberg, 2012. Vol. 142, pp 91-117. |
| [41] | Bohrer F I, Colesniuc C N, Park J, Schuller I K, Kummel A C, Trogler W C. Selective detection of vapor phase hydrogen peroxide with phthalocyanine chemiresistors[J]. J. Am. Chem. Soc., 2008, 130: 3712-3713. |
| [42] | Che Y, Yang X, Liu G, Yu C, Ji H, Zuo J, Zhao J, Zang L. Ultrathin n-type organic nanoribbons with high photoconductivity and application in optoelectronic vapor sensing of explosives[J]. J. Am. Chem. Soc., 2010, 132: 5743-5750. |
| [43] | Zang's group webpage. http:∥www.eng.utah.edu/-lzang/. |
| [44] | Trogler's group webpage. http:∥chem-faculty.ucsd.edu/trogler/. |
| [45] | Swager's group. http:∥web.mit.edu/tswager/www/. |
| [46] | FIDO explosive detector. http:∥gs.flir.com/products/icx-detection/explosives/fido/. |
| [47] | RedX Defense. http:∥www.redxdefense.com. |
| [48] | Vaporsens. http:∥www.vaporsens.com/. |




