端粒是真核细胞线性染色体末端具有(TTAGGG)n重复序列的富含G碱基序列 在细胞分化、DNA复制和维持染色体稳定中起着非常重要的作用[ 1 ]. 正常细胞中端粒长度随着DNA的复制而缩短,当端粒缩短到临界长度时细胞开始凋亡.但癌细胞中独有的端粒酶可以保持端粒长度而使得癌细胞能够无限繁殖.在Na+、K+等一价阳离子存在条件下,(TTAGGG)n序列中的G碱基通过Hoogsteen氢键作用连接形成G-四分体,并进一步堆积形成G-四链体结构[ 2,3 ]. 端粒酶对端粒DNA引物的识别是其链状结构而非特异序列,G-四链体的形成改变了其链状结构,因而不能被RNA模板识别,从而抑制了端粒酶活性的表达,也阻碍了与端粒相关的蛋白质对其靶分子的正常识别,从而达到促进肿瘤细胞死亡的作用[ 4 ].以G-四链体DNA为抗癌药物作用靶点对化合物进行筛选和结构设计是寻找新型抗癌药物的一个重要途径[ 5 ].
配位聚合物是由多齿配体与金属离子配位形成的金属-有机超分子网络结构,在受限的纳米空间里可获得金属离子、配体等构建单元之间的协同作用[ 6,7 ].使得配位聚合物作为功能材料在气体吸附与分离、主-客体超分子化学等领域得到了蓬勃发展[ 8,9 ]. 由于配位聚合物具有良好的生物兼容性,作为药物或药物载体是配位聚合物研究领域的一个新的发展方向[ 10,11,12 ].配位聚合物可通过静电相互作用[ 13 ]、扦插作用[ 14,15,16 ]或沟槽结合[ 17 ]等方式与双链DNA相互作用. DNA与配位聚合物形成的复合物具有优良的光、电性能,可组装成具有特定功能的纳米器件[ 18,19,20 ].到目前为止,有关配位聚合物与G-四链体的相互作用的研究很少.
G-四链体靶向化合物的结构设计主要考虑到增强化合物与G-四分体平面的π-π堆积和与环区碱基或磷酸骨架之间相互作用[ 21,22 ].色胺或色胺酸基团能与DNA碱基进行π-π堆积作用,进而稳定多肽与DNA形成的复合体[ 23 ].异质性细胞核酸蛋白(hnRNP)和牛朊蛋白(BPrP)与G-四链体作用时,其色氨酸的吲哚环与G-四分体可进行π-π堆积,而赖氨酸侧链可与G-四链体具有较强的静电相互作用,使得这些蛋白质能够很好地识别G-四链体[ 24,25 ].色胺修饰竹红菌素及其Y3+、La3+金属离子配位聚合物(M-DTrpHA)具有良好的光、电性能[ 26 ].本论文采用多种光谱手段深入研究M-DtrpHA与G-四链体的相互作用,了解M-DTrpHA与G-四链体的结合模式,希望能进一步拓展配位聚合物在抗癌药物领域中的应用. 1 实验部分 1.1 试剂和仪器
色胺修饰竹红菌素及其Y3+、La3+金属离子配位聚合物参照文献合成[ 26 ].N-(2-羟乙基)哌嗪-N′-2-乙磺酸(HEPES)、色胺 (Trp)、噻唑橙(TO)、小牛胸腺 DNA (CT DNA)均购自Aldrich公司,其余试剂均为分析纯,购自北京化工厂.所有溶剂经无水处理,使用前重蒸.缓冲溶液为用二次蒸馏水配制的NaCl (0.1 mol·L-1) HEPES缓冲溶液(10 mmol·L-1,pH 7.4).
所用仪器: Hitachi U-3100紫外-可见吸收光谱仪,日本; Hitachi F-4600荧光分光光度计,日本; Chirascan圆二色谱仪,英国.
人体端粒序列DNA 22AG (5′-AGGGTTAGGGTTAGGGTTAGGG-3′)由上海生物工程有限公司合成.将22AG溶解在一定量的Milli Q超纯水中,振荡溶解后,在95℃测试260 nm的吸收值,计算22AG的浓度,其中22AG在260 nm处的摩尔消光系数为228500 L·mol-1·cm-1[ 27 ].
CT DNA溶液按照文献的方法配制,并利用CT DNA在260 nm处的吸收确定CT DNA的浓度(摩尔消光系数为6600 L·mol-1·cm-1)[ 28 ] . 1.2 紫外-可见光谱滴定实验
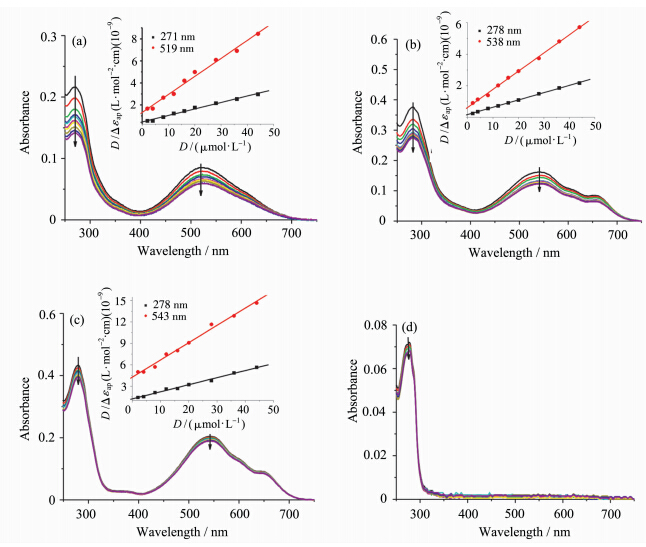
配制5 μmol·L-1的色胺修饰竹红菌素(DTrpHA)及其金属配位聚合物的缓冲溶液2.0 mL,逐渐滴加DNA(初始浓度为1.5 mmol·L-1)溶液,室温静置15 min.采用1 cm石英比色皿,以相应浓度DNA的缓冲溶液为参比,测定DTrpHA及其配位聚合物与DNA混合溶液的紫外-可见吸收光谱. DTrpHA及其配合物与DNA的结合常数可以通过公式(1)求得[ 29 ]:
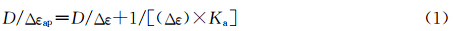
式中D为DNA的浓度.Δεap=∣εa-εf︱,Δε=∣εb-εf︱. εa为化合物的表观摩尔消光系数,εa=Aobs/[化合物],即用加入不同浓度DNA后化合物的吸光度值Aobs除以化合物浓度得到. εf为未加入DNA时,化合物的摩尔消光系数. εb为所有的化合物与DNA结合后的摩尔消光系数. Ka为化合物与DNA的结合常数,用D/Δεap与D作图,利用线性拟合后得到的斜率除以相应的截距,进而求得Ka. 1.3 圆二色谱(CD)光谱实验
在缓冲溶液中加入20 μmol·L-1的G-四链体和20 μmol·L-1的DTrpHA或其配位聚合物,冰箱4 ℃静置过夜后,加入测定池中,在230—320 nm范围内扫描CD光谱图. 扫描速率100 nm/min,带宽10 nm,读数5次,响应时间1 s. 1.4 DNA熔解曲线的测定
缓冲溶液中加入DNA与DTrpHA或其配位聚合物(10 μmol·L-1),室温静置2 h.然后由24 ℃逐渐加热到96 ℃,升温速度控制为1 ℃·min-1,测定紫外吸收光谱.将CT DNA和G-四链体在260和295 nm处的吸收变化值分别进行归一化后对温度作图,中间拐点处的温度即为DNA的熔解温度. 1.5 荧光嵌插剂置换实验(FID)
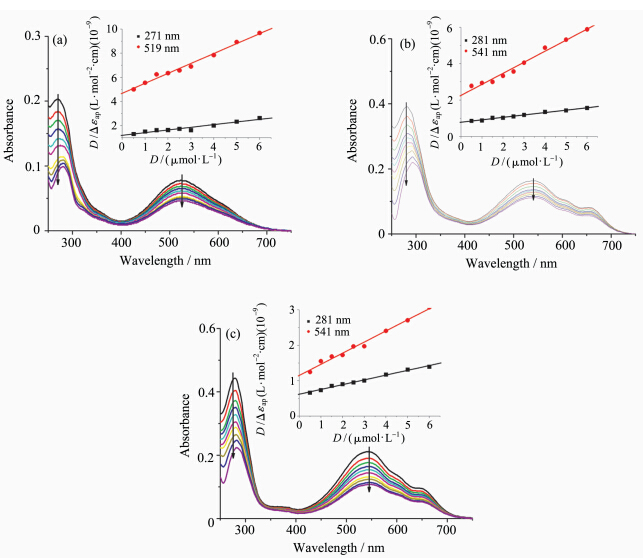
在1 mL缓冲溶液中加入噻唑橙(0.5 μmol·L-1)和DNA(0.25 μmol·L-1),混合均匀后避光静置2 h.再加入适量的DTrpHA或其配位聚合物,使其浓度分别为0.25、0.5、0.75、1.0、1.25、1.5、1.75、2.0、2.5 μmol·L-1,避光静置5 min后进行荧光光谱测试,激发波长为501 nm,扫描范围为510—700 nm.计算出加入化合物前后噻唑橙(TO)的荧光峰面积,采用公式(2)计算噻唑橙的置换百分比.
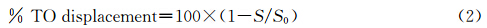
其中S0、S分别为加入化合物前后,TO与DNA混合溶液的荧光峰面积.在G-四链体或双链DNA与TO混合溶液中加入化合物,使TO置换百分比为50%所需化合物的浓度分别为该化合物的G4DC50(使噻唑橙/G-四链体体系荧光下降50%所需配体或配合物的浓度)和dsDC50(使噻唑橙/CT DNA体系荧光下降50%所需配体或配合物的浓度). 2 结果与讨论 2.1 紫外-可见吸收光谱
竹红菌素是一种具有抗肿瘤活性的天然光敏剂,对顺铂类耐药性细胞也具有很强的杀伤作用[ 30,31,32 ]. 利用金属离子与竹红菌素形成配合物或配位聚合物已成为竹红菌素改性的一种重要方法,该方法具有制备简单,能提高竹红菌素的水溶性和对生物大分子的亲合性的优点[ 33,34 ].利用色胺与巯基乙酸双取代反应修饰竹红菌甲素得到色胺修饰竹红菌素DTrpHA. DTrpHA能够与Y3+、La3+等稀土金属离子形成摩尔比为2∶1 ([DTrpHA]/[M3+])的配位聚合物M-DTrpHA (图1).
 | 图1 DTrpHA及其金属配合物的化学结构 Chemical structures of DTrpHA and its coordinaiton polymer M-DTrpHA |
DTrpHA在271 和519 nm有两个吸收峰,可分别归属为色胺基团和竹红菌素基团的特征吸收峰.随着CT DNA的加入,这两个吸收峰的强度逐渐下降(图2a).当CT DNA与DTrpHA的摩尔比为8.8时,其减色效应分别为34.2%和30.5%,表明CT DNA与DTrpHA具有较强相互作用.利用线性拟合作图求得DTrpHA在271和519 nm处的结合常数分别为1.45×105和1.25×105 L·mol -1.
 | 图2 加入CT DNA对(a) DTrpHA,(b) La3+-DTrpHA,(c) Y3+-DTrpHA和 (d) Trp缓冲溶液的紫外-可见吸收光谱的影响 插图为D/Δεap与D作图 UV-Vis absorption spectra of (a) DTrpHA,(b) La3+-DTrpHA,(c) Y3+-DTrpHA and (d) Trp in buffer (0.1 mol·L-1Na+,HEPES (10 mmol·L-1,pH 7.4) upon addition of CT DNA Inset: the plot of D/Δεap vs D [DTrpHA]=[La3+-DTrpHA] = [Y3+-DTrpHA] = [Trp]= 5 μmol·L-1, [CT DNA]=0,2,4,8,12,16,20,28,36,44 μmol·L-1 |
La3+-DTrpHA的吸收峰相对于DTrpHA的吸收峰,均有红移,分别在278和541 nm处.其中,色胺基团的吸收峰红移了7 nm左右,可能是由于色胺基团所处微环境不同引起的[ 35, 36 ],而竹红菌素基团的吸收峰红移了22 nm,可能是由于与稀土离子配位的原因[ 37 ]. CT DNA的加入使得La3+-DTrpHA在278和538 nm处的吸收峰强度均有下降(图2b),当CT DNA与La3+-DTrpHA的浓度比为8.8时,其减色效应分别为26.9%和24.0%.利用线性拟合作图求得La3+-DTrpHA在278和538 nm处的结合常数分别为2.64×105和1.85×105 L·mol-1.
加入CT DNA对Y3+-DTrpHA在278和543 nm处吸收峰的影响较小,说明CT DNA与Y3+-DTrpHA的作用相对较弱(图2c).加入CT DNA后,色胺的吸收光谱仅有微弱变化(< 5%)(图2d).文献报道色胺基团与双链DNA的作用较弱,结合常数为102—103L·mol-1[ 38 ].DTrpHA及其金属配合物与CT DNA的结合常数比较见表1. DTrpHA及其配位聚合物与CT DNA作用过程中,色胺基团和竹红菌素基团的吸收峰强度下降,但未观察到吸收峰红移(表1),表明DTrpHA及其金属配位聚合物与CT DNA的碱基π-π堆积作用较弱,其作用方式可能为沟槽结合[ 17 ].
| 表1 CT DNA对DTrpHA及其金属配位聚合物紫外吸收光谱性质的影响 Absorption spectral properties of DTrpHA and M-DTrpHA bound to CT DNA |
加入G-四链体22AG后,DTrpHA在271和519 nm处的吸收下降(图3a).当22AG与DTrpHA的浓度比为1∶1时,271 nm吸收峰红移至281 nm,其减色效应为55.2%(表2).较大的减色效应和峰位红移表明DTrpHA中色胺基团可能与G-四链体的末端G-四分体具有良好的π-π堆积作用[ 39,40 ].519 nm处的吸收峰随着22AG的加入逐渐下降,至22AG与 DTrpHA的浓度比为1∶1时,其减色效应为40.1%,但其峰位置基本不变(表2).说明DTrpHA中竹红菌素基团与G-四链体22AG中碱基的堆积程度相对较小,可能通过非特异性结合模式与G-四链体环区碱基作用[ 41 ].加入G-四链体22AG使得Y3+-DTrpHA和La3+-DTrpHA中色胺基团的吸收光谱发生红移,具有较大减色效应(图3b、3c),而Y3+-DTrpHA和La3+-DTrpHA中的竹红菌素基团只观测到了吸收峰的下降,吸收峰位未发生变化(表2),表明Y3+-DTrpHA和La3+-DTrpHA中色胺基团也可通过外部π-π堆积作用与22AG结合,而其中的竹红菌素基团与环区碱基或磷酸骨架通过静电作用等非特异性结合模式与G-四链体作用.
 | 图3 加入G-四链体22AG对(a) DTrpHA,(b) La3+-DTrpHA, (c) Y3+-DTrpHA缓冲溶液的紫外-可见吸收光谱的影响 插图为D/Δεap与D作图 UV-Vis absorption spectra of (a) DTrpHA,(b) La3+-DTrpHA,and (c) Y3+-DTrpHA in buffer (0.1 mol·L-1Na+,HEPES 10 mmol·L-1,pH 7.4) upon addition of G-quadruplex 22AG Inset: the plot of D/Δεap vs D [DTrpHA]=[La3+-DTrpHA]=[Y3+-DTrpHA]=[Trp]=5 μmol·L-1, [22AG]=0,0.5,1,1.5,2,2.5,3,4,5,6 μmol·L-1 |
| 表2 G-四链体22AG对DTrpHA及其金属配位聚合物吸收光谱性质影响 Absorption spectral properties of DTrpHA and M-DTrpHA bound to G-quadruplex 22AG |
DTrpHA及其配位聚合物与G-四链体的结合常数与文献报道的聚合酶[ 42 ]或金属配合物[ 43 ]与G-四链体的结合常数相近,约为105mol·L-1左右,表明DTrpHA及其配位聚合物与G-四链体具有较强的相互作用. DTrpHA及其配位聚合物的竹红菌素基团与G四链体22AG作用的结合常数,要大于与双链CT DNA作用的结合常数,可能是由于其结合模式不同引起的.配位聚合物配体的芳香环主要位于主链上,只能通过部分芳香环与刚性结构的双链DNA进行作用[ 44,45,46 ]. G-四链体的环区碱基构象具有一定的柔性,更有利于与配合物或有机配体的相互作用[ 47,48 ]. DtrpHA的稀土离子配位聚合物La3+-DTrpHA和Y3+-DTrpHA能够形成具有纳米孔径的空间网状结构,可包结富勒烯等客体分子[ 26 ]. G-四链体的尺寸与富勒烯相当(~1 nm)[ 3 ],因此G-四链体也可能进入配位聚合物的纳米孔径,并与配位聚合物作用. Y3+-DTrpHA中的色胺基团和竹红菌素基团与22AG作用的结合常数分别是它们与双链CT DNA结合常数的2.70和5.68倍,说明Y3+-DTrpHA与G-四链体作用具有较好的选择性. 2.2 圆二色谱(CD)光谱实验
CD光谱可以有效表征核酸的二级结构,并用于研究DNA与化合物作用前后的构象变化[ 49 ].在0.1 mol·L-1的Na+缓冲溶液中,22AG的CD谱图在265 nm处有一个最大负峰,在246和295 nm处有两个正峰.表明在0.1 mol·L-1的Na+条件下,G-四链体22AG主要以分子内反平行结构存在(图4).[ 50,51 ]加入DTrpHA,Y3+-DTrpHA和La3+-DTrpHA后,22AG的CD谱图峰位没有发生变化,但是其CD峰位强度增强,表明DTrpHA及其配位聚合物能够稳定22AG G-四链体的反平行结构.
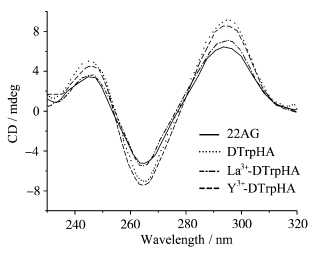 | 图4 加入DTrpHA、La3+-DTrpHA 和Y3+-DTrpHA对含有0.1 mol·L-1Na+HEPES缓冲溶液中 的G-四链体22AG (20 μmol·L-1) 的圆二色谱影响 CD spectra of G-quadruplex 22AG (20 μmol·L-1) in HEPES(10 mmol·L-1,pH 7.4) buffer supplemented with 0.1 mol·L-1Na+upon addition of DTrpHA,La3+-DTrpHA or Y3+-DTrpHA [DTrpHA]=[La3+-DTrpHA]=[Y3+-DTrpHA] = 20 μmol·L-1 |
当化合物具有多个可能结合位点时,化合物与G-四链体作用引起的光谱变化比较复杂,往往需要结合多种方法才能确定化合物不同基团与DNA的作用方式和结合位点.熔解作用是DNA的一个极其重要的物理化学性质.通过测定DNA的熔解温度(Tm),可以进一步了解化合物对DNA稳定性的影响.G-四链体DNA在295 nm处的吸光值会随着温度的变化而变化,可以用来测定G-四链体的熔解温度[ 52 ]. 在含有0.1 mol·L-1 Na+的缓冲溶液中,G-四链体的熔解温度为52.5 ℃(图5a),与文献报道值相近[ 53 ].加入等浓度的DTrpHA、Y3+-DTrpHA、La3+-DTrpHA后,其熔解温度分别升高了6.0、11.0、13.1 ℃(表3),说明DTrpHA及其配位聚合物能够很好地稳定G-四链体.圆二色谱结果表明,加入DTrpHA及其配位聚合物前后,G-四链体22AG的构象均为反平行结构,表明DTrpHA及其配位聚合物存在条件下,22AG的熔解过程相同,均由反平行结构G-四链体转变为单链DNA.紫外吸收光谱结果表明色胺基团能够与G-四分体进行外部堆积作用,但是Y3+-DTrpHA及La3+-DTrpHA带有正电荷,可进一步增强配位聚合物与G-四链体的作用.文献报道具有平面芳香共轭体系且带正电荷的化合物,可与G-四链体的G-四分体通过π-π堆积和静电作用等结合模式来稳定G-四链体的结构[ 21 ],因而对G-四链体的熔解温度影响的顺序为Y3+-DTrpHA>La3+-DTrpHA>DTrpHA.
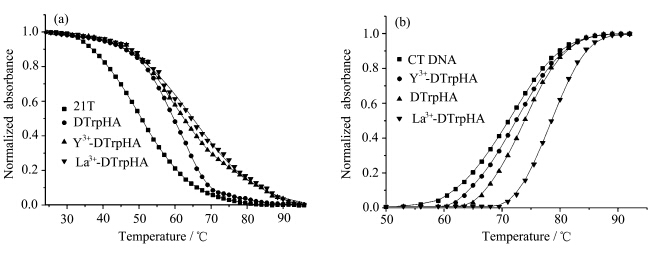 | 图5 0.1 mol·L-1Na+缓冲溶液中,加入DTrpHA、Y3+-DTrpHA、La3+-DTrpHA 对(a) 22AG G-四链体 (10 μmol·L-1)和 (b) CT DNA (50 μmol·L-1)的熔解温度的影响 Normalized melting curve of (a) 22AG G-quadruplex (10 μmol·L-1) and (b) CT DNA (10 μmol·L-1) with the addition of DTrpHA,Y3+-DTrpHA,and La3+-DTrpHA in HEPES buffer containing 0.1 mol·L-1Na+ |
CT DNA的熔解温度为72.1 ℃,加入等浓度的DTrPHA,La3+-DTrpHA及Y3+-DTrpHA后CT DNA的熔解温度分别上升了3.4、6.4、1.9 ℃(图5b).化合物与双链DNA以扦插方式作用时能引起DNA的熔解温度上升大约为10 ℃[ 54 ].CT DNA与DTrpHA及其配位聚合物作用时,没有观察到吸收峰明显的红移现象和减色效应,且DTrpHA及其配位聚合物对CT DNA的熔解温度影响较小 (ΔTm) (表3),上述实验现象均表明DTrpHA及其配位聚合物与CT DNA的结合不是扦插作用,而可能与其他醌类化合物及其配位聚合物相似,采用沟槽结合方式与CT DNA作用[ 55,56 ].
加入Y3+-DTrpHA对G-四链体的熔解温度影响(ΔTm= 13.1 ℃)要明显大于其对双链CT DNA的熔解温度影响(ΔTm=1.9 ℃),表明Y3+-DtrpHA相比DTrpHA和La3+-DtrpHA,与G-四链体具有更强的相互作用.
| 表3 DTrpHA及其配位聚合物对22AG和CT DNA熔解温度的影响 Melting temperature for 22AG and CT DNA in the presence of DTrpHA and its coordination polymersa |
噻唑橙(TO)在缓冲溶液中的荧光较弱,当噻唑橙与双链DNA或G-四链体结合时表现出荧光增强特性.以TO为荧光探针,采用荧光嵌插剂置换法可以比较小分子与DNA的结合强度[ 57,58 ].化合物的DC50值越小,化合物与DNA的亲和性越高.在TO和22AG混合溶液中逐渐加入La3+-DTrpHA和Y3+-DTrpHA,TO的置换百分比增大(图6a),其G4DC50值分别为0.91和0.64 μmol·L-1(图6a).双链CT DNA和TO混合溶液中,加入La3+-DTrpHA可使TO荧光下降,其 dsDC50值为2.40 μmol·L-1(图6b).而加入Y3+-DTrpHA对CT DNA与TO混合溶液的荧光影响较小,未能检测到其DC50值.Trp或DTrpHA对TO和DNA混合溶液的荧光影响较小(图6).在Trp或DTrpHA与TO的浓度比为5的范围内未能检测到dsDC50或G4DC50值,表明Trp和DTrpHA与双链DNA和G-四链体的结合能力都较弱.
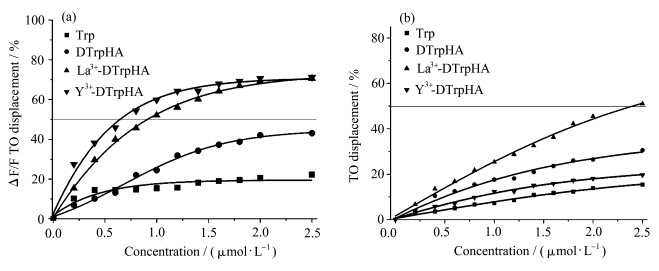 | 图6 Trp、DTrpHA、La3+-DTrpHA和Y3+-DTrpHA在(a) 22AG及(b) CT DNA溶液中的荧光嵌插剂置换曲线 Fluorescent intercalator displacement (FID) curves for Trp,DTrpHA,La3+-DTrpHA and Y3+-DTrpHA with (a) 22AG and (b) CT DNA |
La3+-DTrpHA的dsDC50与G4DC50之比为2.64,对G-四链体与双链DNA的选择性与文献报道的铂类配合物相当[ 59 ] .Y3+-DTrpHA的G4DC50值较小,而其dsDC50值未能检测到,说明Y3+-DTrpHA也具有较高的G-四链体亲和性和选择性,这与紫外-可见光谱以及熔解温度实验结果相一致. FID实验结果进一步表明,将DTrpHA与稀土金属离子形成配位聚合物是提高竹红菌素衍生物与G-四链体的亲和性和选择性的一个有效途径. 3 结论
DTrpHA及其配位聚合物与双链CT DNA和G-四链体的结合模式和作用位点具有较大差异. DTrpHA及其配位聚合物通过沟槽作用与CT DNA结合,但采用外部π-π堆积作用和非特异性结合等多种方式与G-四链体结合. DTrpHA的稀土金属离子配位聚合物能提高G-四链体的熔解温度(ΔTm> 10 ℃),具有较小的G4DC50值,表现出比DTrpHA本身具有更好的G-四链体亲和性和更强的相互作用.
| [1] | Makarov V L, Hirose Y, Langmore J P. Long G tails at both ends of human chromosomes suggest a C strand degradation mechanism for telomere shortening[J]. Cell, 1997, 88(5): 657-666. |
| [2] | Biffi G, Tannahil D, McCafferty J, et al. Quantitative visualization of DNA G-quadruplex structures in human cells[J]. Nature Chemistry, 2013, 5(3): 182-186. |
| [3] | Tagore D M, Sprinz K I, Fletcher S, et al. Protein recognition and denaturation by self-assembling fragments on a DNA quadruplex scaffold[J]. Angewandte Chemie, 2007, 119(46): 227-229. |
| [4] | Bochman M, Paeschke L K, Zakian V A. DNA secondary structures: stability and function of G-quadruplex structures[J]. Nature Reviews Genetics, 2012, 13(11): 770-780. |
| [5] | Georgiades S N, Karim N H A, Suntharalingam K, et al. Interaction of metal complexes with G-quadruplex DNA[J]. Angewandte Chemie, 2010, 49(24): 4020-4034. |
| [6] | Zhang X, Chen Z K, Loh K P. Coordination-assisted assembly of 1-D nanostructured light-harvesting antenna[J]. Journal of the American Chemical Society, 2009, 131(21): 7210-7211. |
| [7] | Fujigaya T, Jiang D L, Aida T. Spin-crossover dendrimers: generation number-dependent cooperativity for thermal spin transition[J]. Journal of the American Chemical Society, 2005, 127(15): 5484-5489. |
| [8] | Spokoyny A M, Kim D, Sumrein A, et al. Infinite coordination polymer nano-and microparticle structures[J]. Chemical Society Reviews, 2009, 38(5):1218-1227. |
| [9] | Ma Z, Moulton B. Recent advances of discrete coordination complexes and coordination polymers in drug delivery[J]. Coordination Chemistry Reviews, 2011, 255(15-16): 1623-1641. |
| [10] | Lou B, He F. Coordination polymers as potential solid forms of drugs: three zinc(II) coordination polymers of theophylline with biocompatible organic acids[J]. New Journal of Chemistry, 2013, 37(2): 309-316. |
| [11] | Zhao D, Tan S, Yuan D,et al. Surface functionalization of porous coordination nanocages via click chemistry and their application in drug delivery[J]. Advanced Materials, 2011, 23(1): 90-93. |
| [12] | Rieter W, Pott J K M, Taylor K M L, et al. Nanoscale coordination polymers for platinum-based anticancer drug delivery[J]. Journal of the American Chemical Society, 2008, 130(35): 11584-11585. |
| [13] | Gimenez J L, Alzuet G, Gonzalez M, et al. Oxidative nuclease activity of ferromagnetically coupledμ-hydroxo-μ-propionato copper(II) complexes [Cu3(L)2(μ-OH)2](L= N-(pyrid-2-ylmethyl) R-sulfonamidato, R=benzene, toluene, naphthalene)[J]. Journal of Inorganic Biochemistry, 2009, 103(0162-0134): 243-255. |
| [14] | Wu H, Jia F, Kou F, et al. A schiff base N-(2-hydroxylacetophenone)-3-oxapentane-1, 5-diamine and its copper(II) coordination polymer: synthesis, crystal structure, antioxidation, and DNA-binding properties[J]. Journal of Coordination Chemistry, 2011, 64(20): 3454-3464. |
| [15] | Gao E, Shi Q, Liu L, et al. Structure, DNA binding studies of complex {[Cu(phen)(py)(H2O)2]) 2H2O}n[J]. Chinese Journal of Chemistry, 2009, 27(12): 2341-2346. |
| [16] | Li Y T, Liu Z Q, Wu Z Y. One-dimensional copper(II) coordination polymers bridged both byμ-trans-oxamidates and phenyldicarboxylates: Synthesis, crystal structure and DNA binding studies[J]. Journal of Inorganic Biochemistry, 2008, 102(9): 1790-1797. |
| [17] | Liu Z Q, Li Y T, Wu Z Y, et al. [Cu4(H2O)4(btc)]n·10nH2O: The first two-dimensional polymeric copper(II) complex with bridging μ-trans-oxamidate and μ4-1,2,4,5-benzentetracarboxylato ligands: Synthesis, crystal structure and DNA binding studies[J]. Inorganica Chimica Acta, 2009, 71(0020-1693): 71-77. |
| [18] | Song K M, Jeong E, Jeon W, et al. Aptasensor for ampicillin using gold nanoparticle based dual fluorescence colorimetric methods[J]. Analytical and Bioanalytical, 2012, 402(6): 2153-2161. |
| [19] | Herrero J G, Zamora F. Coordination polymers for nanoelectronics[J]. Advanced Materials, 2011, 23(44): 5311-5317. |
| [20] | Zhang Y, Luo Y, Tian J, et al. Rectangular coordination polymer nanoplates: largescale, rapid synthesis and their application as a fluorescent sensing platform for DNA detection[J]. PLoS ONE, 2012, 7(1): 30426-30434. |
| [21] | 田明月, 张秀凤, 潘然, 等. 原癌基因c-myc 启动区 G-四链体结构及靶向小分子配体[J]. 化学进展, 2010, 22(5): 983-992. Tian M Y, Zhang X F, Pan R, et al. Proto-oncogene c-myc promoter G-quadruplex structure and targeted small molecule ligands[J]. Progress in Chemistry, 2010, 22(5): 983-992. |
| [22] | Arora A, Kumar N, Agarwal T, et al. Retraction: human telomeric G-quadruplex: targeting with small molecules[J]. FEBS Journal, 2010, 277(5): 1345-1360. |
| [23] | Howell L A, Searcey M. Targeting higher-order DNA: beyond the G-quadruplex[J]. ChemBioChem, 2009, 10(13): 2139-2143. |
| [24] | Mashima T, Matsugami A, Nishikawa F, et al. Unique quadruplex structure and interaction of an RNA aptamer against bovine prion protein[J]. Nucleic Acids Research, 2009, 37(18): 6249-6258. |
| [25] | Dominguez C, Fisette J F, Chabot B, et al. Structural basis of G-tract recognition and encaging by hnRNP F quasi-RRMs[J]. Nature Structural & Molecular Biology, 2010, 17(7): 853-861. |
| [26] | Ou Z Z, Jin H L, GaoY Y, et al. Synthesis and photophysical properties of a supramolecular host guest assembly constructed by fullerenes and tryptamine modified hypocrellin[J]. The Journal of Physical Chemistry B, 2012, 116(7): 2048-2058. |
| [27] | Castor K J, Mancini J, Fakhoury J, et al. Platinum(II) phenanthroimidazoles for targeting telomeric G-quadruplexes[J]. ChemMedChem, 2012, 7(1): 85-94. |
| [28] | Karlsson H J, Eriksson M, Perzon E, et al. Groove-binding unsymmetrical cyanine dyes for staining of DNA: syntheses and characterization of the DNA‐binding[J]. Nucleic Acids Research, 2003, 31(21): 6227-6234. |
| [29] | Wolfe A, Schimer G H, Meehan T. Polycyclic aromatic hydrocarbons physically intercalate into duplex regions of denatured DNA[J]. Biochemistry, 1987, 26(20): 6392-6396. |
| [30] | Lee H Y, Zhou Z X, Chen S, et al. New long-wavelength ethanolamino-substituted hypocrellin: photodynamic activity and toxicity to MGC803 cancer cell[J]. Dyes and Pigments, 2006, 68(1): 1-10. |
| [31] | Zhou Z, Yang H, Zhang Z. Role of calcium in phototoxicity of 2-butylamino-2-demethoxy-hypocrellin A to human gastric cancer MGC-803 cells[J]. Biochimica et Biophysica Acta, 2003, 1593(2-3): 191-200. |
| [32] | 尚立群, 周乃康, 顾瑛, 等. 顺铂耐药及非耐药肺癌细胞对竹红菌乙素的吸收及其光动力杀伤效应的比较[J]. 中国激光医学杂志, 2005, 14(4): 233-238. Shang L Q, Zhou N K, Gu Y, et al. Absorption of hypocrellins B in multidrug-resistant and non-drug-resistant lung cancer cells and their photodynamic killing effect: a comparative study[J]. Chinese Journal of Laser Medicine & Surgery, 2005, 14(4): 233-238. |
| [33] | Zeng Z H, Zhou J H, Zhang Y, et al. Photodynamic properties of hypocrellin A, complexes with rare earth trivalent ions: role of the excited state energies of the metal ions[J]. Journal of Physical Chemistry B, 2007, 111(10): 2688-2696. |
| [34] | Ma J, Zhao J Q, Jiang L. The aluminium(III) complex of hypocrellin B as a PDT photosensitizer[J]. New Journal of Chemistry, 2001, 25(6): 847-852. |
| [35] | Rafferty C N, Mullenberg C G, Shichi H. Tryptophan in bovine rhodopsin: its content, spectral properties, and environment[J]. Biochemistry, 1980, 19(10): 2145-2151. |
| [36] | Steven W L, Thomas P S. Specific tryptophan UV-absorbance changes are probes of the transition of rhodopsin to its active state[J]. Biochemistry, 1996, 35(34): 11149-11159. |
| [37] | Zhou J H, Liu J H, Xia S Q, et al. Effect of chelation to lanthanum ions on the photodynamic properties of hypocrellin A[J]. Journal of Physical Chemistry B, 2005, 109(41): 19529-19535. |
| [38] | Dimicoli J, Helene C. Interactions of aromatic residues of proteins with nucleic acids. I. Proton magnetic resonance studies of the binding of tryptophan-containing peptides to poly(adenylic acid) and deoxyribonucleic acid[J]. Biochemistry, 1974, 13(4): 714-723. |
| [39] | Wang L, Wu Y, Chena T, et al. Novel platinum complexes as efficient G-quadruplex DNA binders and telomerase inhibitors[J]. International Journal of Biological Macromolecules, 2013, 55(1): 185-192. |
| [40] | Kieltyka R, Fakhoury J, Moitessier N, et al. Platinum phenanthroimidazole complexes as G-quadruplex DNA selective binders[J]. Chemistry-A European Journal, 2008, 14(4): 1145-1154. |
| [41] | Suntharalingam K, White A J P, Vilar R. Two metals are better than one: investigations on the interactions between dinuclear metal complexes and quadruplex DNA[J]. Inorganic Chemistry, 2010, 49(18): 8371-8380. |
| [42] | Soldatenkov V A, Vetcher A A, Duka T, et al. First evidence of a functional interaction between DNA quadruplexes and poly(adp-ribose) polymerase-1[J]. ACS Chemical Biology, 2008, 3(4): 214-219. |
| [43] | Wu P, Ma D, Leung C, et al. Stabilization of G-quadruplex DNA with platinum(II) schiff base complexes: luminescent probe and down-regulation of c-myc oncogene expression[J]. Chemistry-A European Journal, 2009, 15(47): 13008-13021. |
| [44] | Yang Y, Yan L, Luo X, et al. Three coordination polymers of 5-aminoisophthalic acid with similar benzimidazole derivative ligands: synthesis, structure and DNA-binding studies[J]. Supramolecula Chemistry, 2012, 24(11): 810-818. |
| [45] | Raja D S, Bhuvanesh N S, Natarajan P K. A novel water soluble ligand bridged cobalt(II) coordination polymer of 2-oxo-1,2-dihydroquinoline-3-carbaldehyde (isonicotinic) hydrazone: evaluation of the DNA binding, protein interaction, radical scavenging and anticancer activity[J]. Dalton Transactions, 2012, 41(15): 4365-4377. |
| [46] | Patra K, Roy S, Chakravarty A R. Synthesis, crystal structures, DNA binding and cleavage activity of l-glutamine copper(II) complexes of heterocyclic bases[J]. Inorganica Chimica Acta, 2009, 362(5): 1591-1599. |
| [47] | Cyrnugelj M, Syket P, Plavec J. Small change in a G-rich sequence, a dramatic change in topology: new dimeric G-quadruplex folding motif with unique loop orientationsp[J]. Journal of the American Chemical Society, 2003, 125(26): 7866-7871. |
| [48] | Parkinson G N, Cuenca F, Neidle S. Topology conservation and loop flexibility in quadruplex drug recognition: crystal structures of inter- and intramolecular telomeric DNA quadruplex drug complexes[J]. Journal of Molecular Biology, 2008, 381(5): 1145-1156. |
| [49] | Kypr J, Kejnovska I, Renciuk D, et al. Circular dichroism and conformational polymorphism of DNA[J]. Nucleic Acids Research, 2009, 37(6): 1713-1725. |
| [50] | Paramasivan S, Rujan I, Bolton P H. Circular dichroism of quadruplex DNAs: applications to structure, cation effects and ligand binding[J]. Methods, 2007, 43(4): 324-331. |
| [51] | Hudson J S, Brooks S C, Graves D E. Interactions of actinomycin D with human telomeric G-quadruplex DNA[J]. Biochemistry, 2009, 48(21): 4440-4447. |
| [52] | 蒋才武, 李炳超, 唐乾利. 双氢杨梅素与G-四链体的相互作用研究[J]. 化学学报, 2007, 65(19): 2159-2162. Jiang C W, Li B C, Tang Q L. Interaction between dihydromyricetin and G-quartet[J]. Journal of the Chinese Chemical Society, 2007, 65(19): 2159-2162. |
| [53] | Wang P, Leung C, Ma D, et al. Structure-based design of platinum(ii) complexes as c-myc oncogene down-regulators and luminescent probes for G-quadruplex DNA[J]. Chemistry-A European Journal, 2010, 16(23): 6900-6911. |
| [54] | Ivanov V I, Minchenkova L E, Schyolkina A K. Different conformations of double-stranded nucleic acid in solution as revealed by circular dichroism[J]. Biopolymers, 1973, 12(1): 89-110. |
| [55] | 殷卫峰, 欧植泽, 高云燕, 等. 1,4-二羟基蒽醌及其Y3+配位聚合物与DNA相互作用研究[J]. 化学学报, 2010, 68(14): 1343-1348. Yin W F, Ou Z Z, Gao Y Y, et al. Interaction of 1,4-dihydroxyanthraquinone and its yttrium coordination polymer with DNA[J]. Journal of the Chinese Chemical Society, 2010, 68(14): 1343-1348. |
| [56] | Breslin D T, Coury J E, Anderson J R, et al. Anthraquinone photonuclease structure determines its mode of binding to DNA and the cleavage chemistry observed[J]. Journal of the American Chemical Society, 1997, 119(21): 5043-5044. |
| [57] | Bertrand H, Monchaud D, Cian A D, et al. The importance of metal geometry in the recognition of G-quadruplex-DNA by metal-terpyridine complexes[J]. Organic & Biomolecular Chemistry, 2007, 5(16): 2555-2559. |
| [58] | Wei Y, Guo L. Binding interaction between polycyclic aromatic compounds and DNA by fluorescence displacement method[J]. Environmental Toxicolgy Chemistry, 2009, 28(5): 940-945. |
| [59] | Suntharalingam K, White A J P, Vilar R. Synthesis, structural characterization, and quadruplex DNA binding studies of platinum(Ⅱ)-terpyridine complexes[J]. Inorganic Chemistry, 2009, 48(19): 9427-9435. |




