2. 河南大学 化学化工学院, 河南 开封 475004
2. College of Chemistry and Chemical Engineering, Henan University, Kaifeng 475004, Henan, P. R. China
二氧化钛是一种传统的光催化材料,具有廉价、无毒、稳定性好、强氧化性等优点,是公认理想的绿色环保材料.它能去除大气和水中的污染物从而解决能源和环境问题,因而引起了研究者们的广泛兴趣.但是,其带隙较宽(3.0—3.2 eV),只能被太阳光中的紫外光激发,而紫外光区只占太阳光总能量的3%—5%,太阳能利用率很低.因此,对TiO2进行改性以扩展其可见光响应便成为众多科研工作者研究的目标.
目前,掺杂是较常用的一种改性手段,其中金属离子的掺杂研究较多[ 1,2,3,4,5,6,7,8,9 ].金属离子掺杂可在半导体表面引入缺陷位置或改变结晶度,影响电子与空穴的复合或改变半导体的激发波长,从而提高TiO2的光催化活性或使其具有可见光催化作用.然而,单一金属离子掺杂的TiO2,其光催化活性往往与制备方法、目标降解物有关,且掺杂样品的热稳定性较差,掺杂的金属离子容易成为电子空穴的复合中心,某些制备方法还需要昂贵的仪器[ 10, 11 ].
2001年,Asahi等首次报道了N掺杂的TiO2具有可见光活性[ 12 ],引起了研究者们对非金属掺杂的广泛兴趣,很多此类研究见诸报道[ 13,14,15,16,17,18,19,20 ],其中N掺杂居多.非金属的掺杂改性将TiO2对光的响应拓展到了可见光区,但是非金属的掺杂也存在一定的缺陷,如:难以获得较高的掺杂浓度,稳定性差等.鉴于单一元素掺杂存在的缺陷,二元共掺杂的研究近年来逐步兴起,如N-La[ 21 ]、Ce-N[ 22 ]、N-Fe[ 23 ]、V-N[ 24 ].二元共掺杂可以弥补单一元素掺杂存在的缺陷.Iliev等[ 25 ]采用sol-gel法制备了N掺杂TiO2,然后利用光还原法在其表面负载Au,结果表明,Au/N共掺杂的TiO2 活性最高.Li等[ 26 ]采用浸渍法制备得到Pt/TiO2-xNx,研究的结果表明Pt、N协同作用提高了可见光下催化剂降解有机污染物的能力.Hua等[ 27 ]采用溶胶凝胶法制备了N掺杂TiO2,然后采用光还原沉积法在其表面负载Pt得到Pt、N共掺杂TiO2,结果表明Pt、N共掺杂进一步提高了光催化反应性能.综上可见共掺杂更有利于可见光催化活性的提高.但是,到目前为止,有关N、Bi共掺杂TiO2的报道极为少见[ 28 ].本文以纳米管钛酸(NTA)为Ti的前驱体,以Bi(NO3)3·5H2O为Bi源和N源,采用水热法制备了N、Bi共掺杂的TiO2,该催化剂在可见光下表现出了较高的催化性能. 1 实验部分 1.1 样品的制备
前驱体纳米管钛酸的制备以NaOH和P25为原料,根据文献[ 29, 30 ]进行制备,方法如下:商品TiO2(Degussa P25)多晶粉体与10 mol/L的氢氧化钠(AR,天津科密欧)溶液于高压反应釜中120 ℃水热反应24 h后,自然冷却至室温,所得产物用3次水洗涤至pH为7—8后,用0.1 mol/L的盐酸(36%—38%,AR,洛阳昊华)溶液浸泡5—10 h,再用3次水洗涤至检测不到Cl-,抽滤后室温真空下干燥得到纳米管钛酸(分子式H2Ti2O4(OH)2),记作NTA.
共掺杂样品的制备是以Bi(NO3)3·5H2O为Bi源和N源,取1 g的NTA和适量的硝酸酸化的Bi(NO3)3·5H2O(1 mol/L)溶液,室温搅拌下分散于去离子水中,控制Bi/Ti摩尔比分别为0、0.5%、1%、2%和5%,总体积为50 mL,然后转移至100 mL的高压反应釜中,130 ℃水热反应3 h,冷却至室温,然后用3次水进行洗涤,所得样品标记为N,Bi-130-0、 N,Bi-130-1/2、N,Bi-130-1、N,Bi-130-2和N,Bi-130-5.然后控制Bi/Ti摩尔比为1%,分别于160 ℃和210 ℃,同样恒温3 h,所得样品标记为:N,Bi-160-1和N,Bi-210-1. 1.2 催化剂的表征
使用荷兰Philips X’Pert Pro X射线衍射仪(XRD) 对样品晶型结构进行测试,测试条件为 CuKα辐射,工作电压为40 kV,电流为40 mA;扫描范围为 5°—90°,扫描速度为0.04 °/s.使用日本株式会社JEM-2010 型电子显微镜(TEM) 对样品进行形貌观察,加速电压200 kV;用日本岛津U-3010紫外-可见扩散反射谱仪(UV-Vis DRS) 测定原料和样品的光吸收能力,空白对比为硫酸钡;用英国AXIS ULTRA X射线光电子能谱(XPS),以Al靶单色化的X射线源对样品进行了元素分析,C峰校正为284.8 eV. 1.3 催化剂的可见光活性评价
以甲基橙(MO)为模型反应物研究了样品在可见光下的催化性能.移取3 mL催化剂悬浊液(0.06 g/L)到比色皿反应器中(1.0 cm × 1.0 cm × 4.5 cm) (内放一个小的磁子进行磁力搅拌),加入50 μL甲基橙(0.1 mmol·L-1)溶液,暗态下磁力搅拌30 min,达到吸附脱附平衡,加光后,每隔0.5 h测定一次甲基橙的吸光度,其最大吸收波长为464 nm.光源为 300 W的 Xe灯,光束通过λ≥420 nm截止滤光片,得到光强为43 mW/cm2的可见光[ 31 ]. 2 结果与讨论 2.1 样品的形貌分析
图1为以NTA为前驱体,Bi(NO3)3·5H2O为Bi源和N源,水热法制备的N、Bi共掺杂TiO2样品的形貌图.图1a是前驱体NTA的TEM图,可以看出,NTA是一维的纳米管状结构,内径3—5 nm,外径8—10 nm,管长可达数百纳米乃至微米[ 30 ].130℃水热时,纳米管钛酸(NTA)管状结构已经破坏,由管状转化为颗粒状,如图1b所示.所有的样品都表现出稍欠规则的菱形片状结构,颗粒大小比较均一,粒径在10—20 nm.颗粒呈现菱形可能与反应介质呈酸性有关,为避免硝酸铋水解,反应介质呈酸性,同时为使反应条件相同,未掺杂样品也加了等量的酸,因此部分颗粒也出现菱形片状结构.郑燕青等曾就二氧化钛晶粒的水热制备及其形成机理进行研究,认为前驱物种类和水热反应介质酸碱性决定了产物物相,酸性条件下水热的产物应为菱形片状锐钛矿结构[ 32 ].随着掺杂量的增加,样品出现了团聚现象,而水热温度对样品的粒径和形貌影响不大(此处未给出TEM图像).
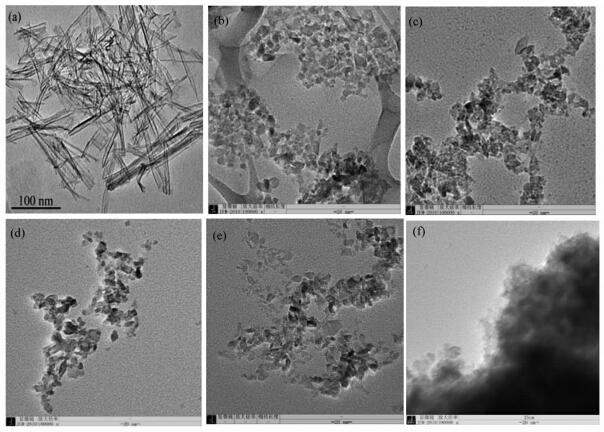 | 图1 氮、铋共掺杂TiO2 的TEM图像 TEM images of the N,Bi-codoped TiO2 samples (a)NTA,(b)N,Bi-130-0,(c)N,Bi-130-1/2,(d)N,Bi-130-1,(e)N,Bi-130-2,(f)N,Bi-130-5 |
图2为N、Bi共掺杂TiO2在不同掺杂量及不同水热条件时的XRD图谱.由图可以看出,所得N、Bi共掺杂样品均以锐钛矿相为主要晶相.然而,随掺杂量增加至Bi/Ti=2%、5%(摩尔分数),在2θ=31°处,有一个微弱的衍射峰可以被观察到,这表明有新的晶相产生,考虑到Bi3+的离子半径(103 pm)比Ti4+的离子半径(67 pm)大很多,若Bi3+取代TiO2晶格中Ti4+,必然引起晶格的畸变,反映到XRD中TiO2特征峰会向高衍射角偏移,而图中并未观察到TiO2特征峰的偏移,因此可以排除Bi3+取代TiO2晶格中Ti4+的可能性.结合文献[ 33 ],所观察到的微弱的衍射峰可能是Bi2O3,这说明Bi3+并没有进入TiO2晶格取代Ti4+,而是以Bi2O3的形式存在.水热温度对样品的结晶度及晶相没有太大影响.
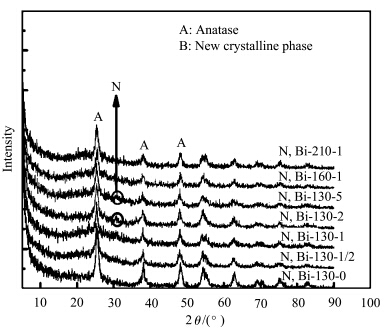 | 图2 氮、铋共掺杂TiO2样品的XRD 图谱 The XRD patterns of N,Bi-codoped TiO2 samples |
图3为所制备样品的紫外可见漫反射(DRS)光谱.从图中可以看出,相对于P25-TiO2,N、Bi共掺杂TiO2在可见光区都表现出了较强的吸收(380—800 nm),但是随着掺杂量的增加,水热温度的升高,样品的可见光吸收强度并没有表现出明显升高的趋势,反而略微下降,尤其是样品N,Bi-130-0,也就是纯的TiO2对可见光的吸收能力超过了所有的铋掺杂样品,这可能是源于样品制备过程中所使用的前驱体纳米管钛酸(NTA)在水热过程中产生了束缚单电子的氧空位,而氧空位在价带和导带之间形成了子能级,从而使TiO2在可见光区产生吸收[ 34 ].共掺杂样品的可见光吸收没有增强反而出现下降趋势,这表明N、Bi掺杂剂的存在会抑制样品对可见光的吸收能力.但是值得注意的是,N、Bi共掺杂样品的光催化活性相对于纯的TiO2却得到了很大提高(参看活性评价部分),这表明光催化剂的光催化活性高低与样品对可见光的吸收能力并没有直接的关系.
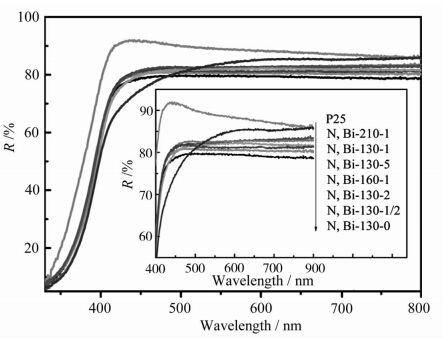 | 图3 氮、铋共掺杂TiO2 的DRS图谱 The DRS of the N,Bi-codoped TiO2 samples |
图4为N、Bi共掺杂TiO2在不同水热温度和不同掺杂量条件下的XPS图谱.图4a、b为N1s的XPS谱图,由图可以看出,所有的掺杂样品都存在两个主要的特征峰,分别位于400.0 eV和407.2 eV.根据文献报道,位于400.0 eV的峰是以间隙态形式存在的氮(Ti—O—N键)[ 35, 36 ];而407.2 eV处的峰则可能来源于硝酸盐中的N1s峰[ 37 ],这表明可能有部分的Bi物种以硝酸盐形式存在,可能是BiONO3,来源于Bi(NO3)3的水解.且由图可以看出,随着掺杂量的增加,400.0 eV处的峰强逐渐减弱,当Bi/Ti摩尔比增至5%时,该处的峰消失,这表明Bi含量的增加会对N的存在形式产生影响,进而影响光催化活性.图4c、d为Bi4f的XPS图谱,可以看出,在165 eV和159.7 eV附近有两个吸收峰,这两个特征峰分别对应于Bi3+的Bi4f5/2和Bi4f7/2,表明Bi物种的价态为3价,两个峰之间的距离为5.3 eV[ 38, 39 ],随着Bi/Ti摩尔比提高,Bi3+的特征峰增强.结合XRD分析,可以得出结论,Bi物种主要以Bi2O3和BiONO3两种形式存在.
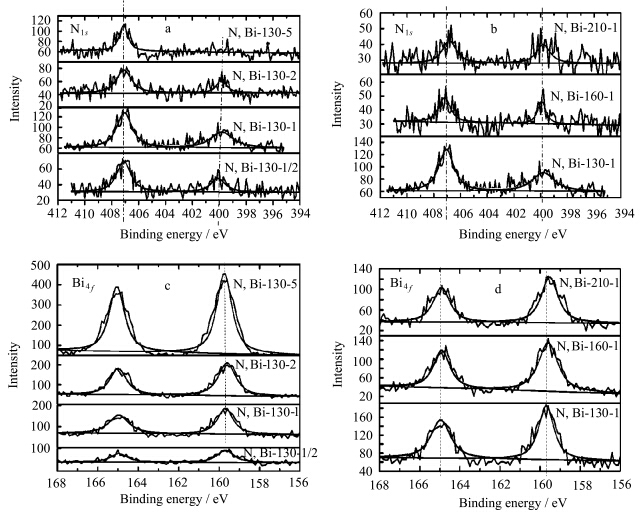 | 图4 氮、铋共掺杂TiO2 的XPS图谱 (a)不同铋掺杂量样品的N1s,(b)不同水热温度样品的N1s,(c)不同铋掺杂量样品的Bi4f,(d)不同水热温度样品的Bi4f The XPS of N,Bi-codoped TiO2 (a) N1s of the samples with different dopant of Bi,(b) N1s of the samples with different hydrothermal temperature, (c) Bi4f of the samples with different dopant of Bi and (d) Bi4f of the samples with different hydrothermal temperature |
图5是样品以甲基橙(MO)为模型反应物在可见光下的脱色效果,可以看出,N,Bi-130-1光催化效果最好,这表明最佳的水热温度是130 ℃,最佳掺杂量是Bi/Ti摩尔比为1%.如前所述,N和Bi的掺杂没有提高样品的可见光吸收,但是光催化活性却得到了提高,这表明N和Bi的存在提高了对光的利用效率,有效抑制了光生电子-空穴对的复合.当然不能排除少量的Bi2O3可能和TiO2形成复合光催化剂有关.而对于有较强可见光吸收的样品N,Bi-130-0,并没有表现出优越的光催化性能,这同光生电子空穴对有较快的复合速率有关.随着掺杂量的增加,样品的光催化活性呈现下降趋势,可能是由于随着掺杂量的增加,BiONO3的量增加,覆盖于TiO2的表面,阻碍了污染物在催化剂表面的吸附.样品的催化活性顺序为N,Bi-130-1 > N,Bi-130-1/2 > N,Bi-130-2> N,Bi-130-5 > N,Bi-130-0 > P25.而随着温度的升高,样品的催化活性出现降低趋势.
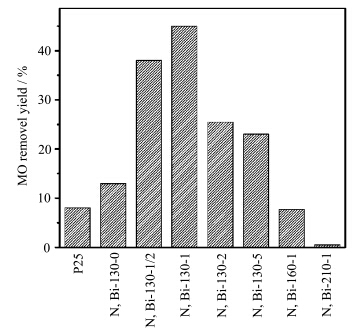 | 图5 氮、铋共掺杂TiO2可见光条件下,催化降解甲基橙脱色过程,3 h降解率 degradation efficiency of MO solution in 3 hours under visible light irradiation |
本文以纳米管钛酸NTA为前驱体,Bi(NO3)3·5H2O为N源和Bi源,采用水热法制备了N、Bi共掺杂的TiO2,考察了掺杂量及水热温度对可见光催化降解MO的活性影响.结果表明所得样品主要为锐钛矿晶型,含有少量的Bi2O3,样品的形貌为菱形片状结构,粒径在20 nm左右.XPS显示,掺杂过程中有少量副产物BiONO3产生,其生成量主要取决于掺杂量.N元素除了以NO-3的形式存在于BiONO3中外,还有少量N在TiO2中形成间隙掺杂氮(Ti—O—N键).水热温度为130℃,掺杂量为1%(摩尔比)时,得到的样品N,Bi-130-1光催化活性最高.催化活性的提高源于N和Bi的掺杂增加了对可见光的利用效率,降低了电子空穴的复合速率.
| [1] | Choi W, Termin A, Hoffmann M R. The role of metal ion dopants in quantum-sized TiO2: correlation between photoreactivity and charge carrier recombination dynamics[J]. The Journal of Physical Chemistry, 1994, 98(51): 13669-13679. |
| [2] | 吴树新, 马 智, 秦永宁,等. 过渡金属掺杂二氧化钛光催化性能的研究[J].感光科学与光化学, 2005, 23(2): 94-101. Wu S X, Ma Z, Qin Y N, et al. Study on photocatalytic activity of transition metal oxide doped TiO2 photocatalysts[J]. Photographic Science and Photochemistry, 2005, 23(2): 94-101. |
| [3] | Wang Y Q, Cheng H M, Zhang L, et al. The preparation, characterization, photoelectrochemical and photocatalytic properties of lanthanide metal-ion-doped TiO2 nanoparticles[J]. Journal of Molecule Cataysis A: Chemical, 2000, 151(1-2): 205-216. |
| [4] | Yamashita H, Harada M, Misaka J, et al. Degradation of propanol diluted in water under visible light irradiation using metal ion-implanted titanium dioxide photocatalysts[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2002, 148(1-3): 257-261. |
| [5] | Li F B, Li X Z, Hou M F, et al. Enhanced photocatalytic activity of Ce3+-TiO2 for 2-mercaptobenzothiazole degradation in aqueous suspension for odour control[J]. Applied Catalysis A: General, 2005, 285(1-2): 181-189. |
| [6] | Choi J, Park H, Hoffmann M R. Effects of single metal-ion doping on the visible-light photoreactivity of TiO2[J]. The Journal of Physical Chemistry C, 2010, 114(2): 783-792. |
| [7] | Wu J C S, Chen C H. A visible-light response vanadium-doped titania nanocatalyst by sol-gel method[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2004, 163(3): 509-515. |
| [8] | Wu Q P, Zheng Q, Krol R van de. Creating oxygen vacancies as a novel strategy to form tetrahedrally coordinated Ti4+ in Fe/TiO2 nanoparticles[J]. The Journal of Physical Chemistry C, 2012, 116(12): 7219-7226. |
| [9] | Fan X X, Chen X Y, Zhu S P, et al. The structural, physical and photocatalytic properties of the mesoporous Cr-doped TiO2[J]. Journal of Molecule Cataysis A: Chemical, 2008, 284(1-2): 155-160. |
| [10] | Takeuchi M, Yamashita H, Matsuoka M, et al. Photocatalytic decomposition of NO under visible light irradiation on the Cr-ion-implanted TiO2 thin film photocatalyst[J]. Catalysis Letters, 2000, 67(2-4): 135-137. |
| [11] | Anpo M. Use of visible light. Second-generation titanium oxide photocatalysts prepared by the application of an advanced metal ion-implantation method[J]. Pure and Applied Chemistry, 2000, 72(9): 1787-1702. |
| [12] | Asahi R, Morikawa T, Ohwaki T, et al. Visible-light photocatalysis in nitrogen-doped titanium oxides[J]. Science, 2001, 293(5528): 269-271. |
| [13] | Valentin C D, Pacchioni G, Selloni A, et al. Characterization of paramagnetic species in N-doped TiO2 powders by EPR spectroscopy and DFT calculations[J]. The Journal Physical Chemistry B, 2005, 109(23): 11414-11419. |
| [14] | Ohno T, Murakami N, Tsubota T, et al. Development of metal cation compound-loaded S-doped TiO2 photocatalysts having a rutile phase under visible light[J]. Applied Catalysis A: General, 2008, 349(1-2): 70-75. |
| [15] | Kang I C, Zhang Q W, Yin S, et al. Preparation of a visible sensitive carbon doped TiO2 photo-catalyst by grinding TiO2 with ethanol and heating treatment[J]. Applied Catalysis B: Environmental, 2008, 80(1-2):81-87. |
| [16] | Li D, Haneda H, Labhsetwar N K, et al. Visible-light-driven photocatalysis on fluorine-doped TiO2 powders by the creation of surface oxygen vacancies[J]. Chemical Physics Letters, 2005, 401(4-6): 579-584. |
| [17] | Joung S, Amemiya T, Murabayashi M, et al. Low temperature preparation and visible light photocatalytic activity of mesoporous carbon-doped crystalline TiO2[J]. Applied Catalysis B: Environmental, 2007, 69(3-4): 138-144. |
| [18] | Luo H M, Takata T, Lee Y, et al. Photocatalytic activity enhancing for titanium dioxide by co-doping with bromine and chlorine[J]. Chemistry of Materials, 2004, 16(5): 846-849. |
| [19] | Liu G, Chen Z G, Dong C L, et al. Visible light photocatalyst:iodine-doped mesoporous titania with a bicrystalline framework[J]. The Journal Physical Chemistry B, 2006, 110(42):20823-20828. |
| [20] | Li F F, Jiang Y S, Xia M S, et al. Effect of the P/Ti ratio on the visible-light photocatalytic activity of P-doped TiO2[J]. The Journal Physical Chemistry C, 2009, 113(42): 18134-18141. |
| [21] | 陈永刚, 刘素文, 冯光建, 等. 氮镧共掺杂TiO2 纳米粉的溶剂热法制备及其可见光催化性能[J]. 硅酸盐通报, 2009, 28(1): 63-66,75. Chen Y G, Liu S W, Feng G J, et al. Preparation of N, La-doped TiO2 nanopowder by sovolthermal method and its catalytic properties of visible light[J]. Bulletin of the Chinese Ceramic Society, 2009, 28(1): 63-66,75. |
| [22] | Wang C, Ao Y H, Wang P F, et al. Preparation of cerium and nitrogen co-doped titania hollow spheres with enhanced visible light photocatalytic performance[J]. Powder Technology, 2011, 210(3): 203-207. |
| [23] | 刘万兵,邓 健,赵玉宝,等. 铁氮共掺杂纳米TiO2复合膜的制备、光谱分析及光催化活性研究[J]. 光谱学与光谱分析.2009, 29(5): 1394-1397. Liu W B, Deng J, Zhao Y B, et al. Preparation, spectral analysis and photocatalytic activities of TiO2 films codoped with iron and nitrogen[J]. Spectroscopy and Spectral Analysis, 2009, 29(5): 1394-1397. |
| [24] | Gu D E, Yang B C, Hu Y D. V and N co-doped nanocrystal anatase TiO2 photocatalysts with enhanced photocatalytic activity under visible light irradiation[J]. Catalysis Communications, 2008, 9(6): 1472-1476. |
| [25] | Iliev V, Tomova D, Rakovsky S. Nanosized N-doped TiO2 and gold modified semiconductors -photocatalysts for combined UV-visible light destruction of oxalic acid in aqueous solution[J]. Desalination, 2010, 260(1-3): 101-106. |
| [26] | Li D Z, Chen Z X, Chen Y L, et al. A new route for degradation of volatile organic compounds under visible light: using the bifunctional photocatalyst Pt/TiO2-xN<em>x in H2O2 atmosphere[J]. Environmental Science & Technology, 2008, 42(6): 2130-2135. |
| [27] | 华南平, 吴遵义, 杜玉扣, 等. Pt、N共掺杂TiO2在可见光下对三氯乙酸的催化降解作用 [J]. 物理化学学报, 2005, 21(10): 1081-1085. Hua N P, Wu Z Y, Du Y K, et al. Titanium dioxide nanoparticles codoped with Pt and N for photodegradation of Cl3CCOOH[J]. Acta Physico-Chimica Sinica, 2005, 21(10): 1081-1085. |
| [28] | Kang Q M, Yuan B L, Xu J G, et al. Synthesis, characterization and photocatalytic performance of TiO2 codoped with bismuth and nitrogen[J]. Catalysis Letters, 2011, 141(9): 1371-1377. |
| [29] | Zhang S L, Zhou J F, Zhang Z J, et al. Morphological structureand physicochemical properties of nanotube TiO2[J]. Chinese Science Bulletin, 2000, 45(16): 1533-1536. |
| [30] | Yang J J, Jin Z S, Wang X D, et al. Study on composition, structure and formation process of nanotube Na2Ti2O4(OH)2[J]. Dalton Transactions, 2003, 20: 3898-3901. |
| [31] | Han X G, Kuang Q, Jin M S, et al. Synthesis of titania nanosheets with a high percentage of exposed (001) facets and related photocatalytic properties[J]. Journal of America Chemistry Society, 2009, 131(9): 3152-3153. |
| [32] | 郑燕青,施尔畏,元如林,等. 二氧化钛晶粒的水热制备及其形成机理研究[J].中国科学E辑, 1999, 29(3): 206-213. Zheng Y Q, Shi E W, Yuan R L, et al. Study on hydrothermal preparation and formation mechanism of Titania grains[J]. Science in China (Series E), 1999, 29(3): 206-213. |
| [33] | Lv K, Zuo H S, Sun J, et al. (Bi, C and N) codoped TiO2 nanoparticles[J]. Journal of Hazardous Materials, 2009, 161(1): 396-401. |
| [34] | Cui G J, Xu Z X, Wang Y, et al. A new method to prepare the novel a natase TiO2[J]. Surface Review and Letters, 2008, 15(4): 509-513. |
| [35] | Irie H, Watanabe Y, Hashimoto K. nitrogen-concentration dependence on photocatalytic activity of TiO2-xNx powders[J]. The Journal of Physical Chemistry B, 2003, 107(23): 5483-5486. |
| [36] | Sato S, Nakamura R, Abe S. Visible-light sensitization of TiO2 photocatalysts by wet-method N doping[J]. Applied Catalysis A: General, 2005, 284(1-2): 131-137. |
| [37] | Honda F, Hirokawa K. A photoelectron spectroscopic observation of iron surfaces exposed to N2, N2O, NO, NO2, and air at 200 torr or 1 atm[J]. Journal of Electron Spectroscopy and Related Phenomena, 1976, 8(3): 199-211. |
| [38] | Zhou J K, Zou Z G, Ray A K, et al. Preparation and characterization of polycrystalline bismuth titanate Bi12TiO20 and its photocatalytic properties under visible light irradiation[J].Industry & Engineering Chemistry Research, 2007, 46(3):745-749. |
| [39] | Wang Y, Wang Y, Meng Y L, et al. A highly efficient visible-light-activated photocatalyst based on bismuth- and sulfur-codoped TiO2[J]. The Journal Physical Chemistry C, 2008, 112(17): 6620-6626. |




