在过去的几十年间,光聚合反应得到极为广泛的关注和深入的研究.由于具有聚合条件温和、反应速度快、不需要使用溶剂和引发反应易于通过光照进行控制等优点,光聚合反应在涂料、胶黏剂、油墨等传统领域以及骨科和齿科修复材料、光刻胶、3D打印等高新技术领域均得到了广泛的应用[1,2,3,4,5].传统意义上的光聚合均发生在液态条件下,液态环境赋予了单体和引发剂分子良好的运动性,确保可以在短时间内获得较高的官能团转化率.通常认为,由于在固态条件下分子运动高度受限,固态条件下的光聚合反应难以进行.然而,一些研究表明,常见的光聚合单体如TMPTA、HDDA等在聚合物基体(如聚甲基丙烯酸甲酯、聚氯乙烯、聚氨酯、天然橡胶等)所形成的固态环境中仍可获得满意的双键转化率,并可提高聚合物基体的性能[6,7,8].
在最近的30年间,更多的研究关注于实现更为困难的晶态单体的光聚合.不同于液态光聚合,结晶态单体的排列是规整的,因此,这种固态光聚合展示了不同于传统光聚合的许多引人注目的特性.为此,本文将综述已有的关于结晶态单体的固态光聚合方面的工作,并对聚合原理和应用加以介绍. 1固态光聚合体系
迄今为止,能实现固态光聚合的结晶态单体种类仍十分有限.对于研究最为透彻的双炔体系和二烯体系来说,起始化合物的反应活性和最终产物的结构都由反应物晶体中 分子的几何性质决定,反应通过最小的原子和分子运动完成.在这种情况下,反应通常由晶格控制,聚合可以通过晶型转变而完成.由于这些内涵都符合拓扑化学反应的概念,因此,这类固态光聚合也被称为拓扑化学聚合[9, 10].在很多情况下,拓扑化学聚合的一个好处在于可以获得难以制备的聚合物单晶[11].近些年来,一些新的聚合体系被发现也可实现固态光聚合,包括光聚合最为常用的(甲基)丙烯酸酯体系. 1.1 双炔体系
双炔类单体的固态光聚合最早由Wegner等[12]发现.双炔类单体固态光聚合的机理也由Wegner等进行了较为清楚的描述.双炔进行固态光聚合的基本前提条件是,单体分子以晶面间距d=0.47—0.52 nm和分子轴/堆叠轴的夹角约45°的结构进行堆砌[13].双炔通过1,4加成的链式反应进行聚合,生成全反式烯-炔交替聚合物链,如图1所示.组装体或晶体中某一分子的双炔基团一旦吸收一个光子后,在基团两端会产生未配对的双激发态自由基.自由基与相邻的双炔基团发生热加成.得到的二聚体在两端仍然具有能够导致链增长的活性自由基.有实验(ESR)证明,当聚合物链超过五个重复单元以后,卡宾也参与引发了聚合反应[14, 15].
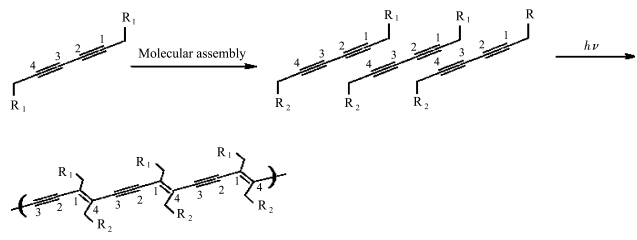 | 图1 双炔的紫外光聚合示意图[13] Schematic representation of the polymerization of assembled functional diacetylenes[13] |
双炔晶体(如己-3,5-双炔-1,6-二醇和其它衍生物)不仅在基础研究方面,也在应用方面扩展至其它领域,促进了相关学科和技术的发展[16,17,18,19,20,21,22,23,24,25,26,27,28].双炔的固态光聚合可以应用于制备Langmuri-Blodgett膜[29]、脂质体、囊泡以及在金属氧化物或石墨表面组装形成单层膜[30,31,32,33].一个典型的例子是二十五烷双炔酸和二十九烷双炔酸在石墨表面形成的有序单分子层的光聚合.聚合后的单层结构引人注目的特性是:伴随着温度、pH或压力等条件的变化,共轭聚双炔构象出现相应变化时,会产生由蓝色到红色的颜色改变,这一现象被用于构筑各种传感器的器件[31,32,33].传感器通过共价键链接到功能化双炔的分子层,与生物体结合会产生一个机械刺激,导致层中聚双炔的构象变化(侧链混乱或主链堆叠的紊乱),光谱随之发生位移.这个方法已用于流行性感冒病毒和霍乱病毒以及葡萄糖一类的生物化学培养基的直接色谱检验. 1.2 二烯体系
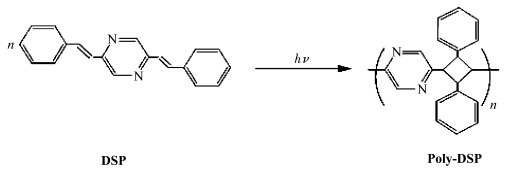
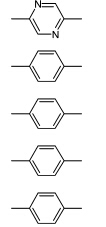
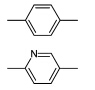
二烯单体的固态光聚合最早的工作可以追溯到Schmidt对肉桂酸及其衍生物二聚反应进行研究的工作[34].时至今日,更多类型的[2+2]反应在Schmidt的开创性工作下得以进行.二烯单体的光聚合是在2,5-二苯乙烯基吡嗪晶体(DSP)的[2+2]光聚合过程中发现的[35,36].DSP的固态光聚合的示意图如图2所示,二烯单体固态光聚合又被称为四中心聚合.随后,具有与DSP类似结构的许多二烯类的单体均被证明可以发生固态光聚合,一些代表性的单体结构如表1所示[37,38,39,40,41,42,43].
| 表1 能够发生四中心体聚合的二烯单体的实例 Examples of diolefins which undergo four-center-photopolymerization |
Hasegawa等[44]在1983年的综述性文章对这类发生[2+2]光聚合反应的二烯单体进行了总结.二烯类单体的聚合机理是逐步聚合,而不是链式反应,换言之,每一个重复单元加到链上都需要进一步吸收光子.尽管这种二烯类的固态光聚合早在上世纪60年代就有报道,然而时至今日,可以发生这种[2+2]光聚合的单体种类仍十分有限.
1,3-丁二烯的衍生物是另一类十分重要的可进行晶态光聚合的二烯类单体[10].由于这类晶态单体在聚合过程中结晶结构不发生破坏并且可以制备具有高度立构规整的结晶聚合物,因此近20年来1,3-丁二烯的衍生物得到了广泛的研究[45,46,47,48,49,50,51,52].关于这类单体的报道最早可以追溯到1994年Mastsumoto对二乙基(Z,Z)粘康酸酯(EMU)的研究[53]:EMU在液态下无法聚合,然而通过晶态光聚合可以得到具有极高立构规整性的聚合物晶体,并且EMU的聚合物有很高的分子量.XRD和ESR的研究表明EMU的晶态光聚合为自由基引发的链式聚合,其链引发过程如图3所示.晶态单体的排列方式对链式聚合的活性有很大的影响:当单体采用柱状结构堆叠并且堆叠距离为0.5 nm[54],可以很好地发生晶态光聚合.随后对粘康酸的多种衍生物(包括酯类和铵盐)的研究表明,通过弱的分子间作用力(包括氢键网络、芳环的堆叠以及CH/π或卤素与卤素的弱氢键作用)可以有效地使粘康酸的衍生物在结晶状态下形成圆柱形堆积,且堆积距离接近0.5 nm.这样,通过对单体晶体结构的设计,粘康酸的多种衍生物均可以实现晶态光聚合反应.
 | 图3 EMU晶态光聚合机理的研究[55] Mechanism of photopolymerization of diethyl (Z,Z)-2,4-hexadienedioate (diethyl cis,cis-muconate) (EMU) in the crystalline state[55] |
粘康酸的衍生物的晶态光聚合产物的分子量和立构规整性一直是研究者们所关注的.在分子量方面,粘康酸衍生物的晶体在光聚合后得到高分子量的聚合物(Mn=(1—4)×105).聚合物的分子量由初始晶体的尺寸控制:随着单体晶体初始尺寸的降低,分子量也随之降低[10,56].在立构规整性方面,得到了一系列具有不同立体结构的高度规整的结晶聚合物.单体分子的排列方式对聚合物的立构规整性有很大的影响.如图4所示,分子平行堆叠的(E,Z)-丁二烯衍生物(例如萘亚甲基铵(E,Z) 粘康酸盐)可以聚合得到外消旋双全同立构的聚合物,而分子平行堆叠的(E,E)-或(Z,Z)-丁二烯衍生物可以得到内消旋双全同立构的聚合物[57].尽管大多数丁二烯衍生物分子采用平行堆叠的方式得到双全同立构的聚合物,但是通过引入CH/π的弱氢键作用,如双(4-取代苄基) 粘康酸酯,可以获得交替堆叠的分子排列方式,从而得到双间同立构的聚合物[58].需要说明的是,尽管晶态光聚合在控制聚合物的立构规整性具有明显的优势,然而,这类光聚合体系仍局限于粘康酸衍生物体系.
 | 图4 丁二烯衍生物单体排列方式与聚合物立体结构的关系 (a)单体分子平行排列; (b) 单体分子交替排列[57] Relationship between the crystal packing of monomer molecules with different configurations and the stereochemical structure of polymers produced during topochemical polymerization of 1,4-disubstituted 1,3-butadienes (a) Translational molecular packing in the column;(b) Alternating molecular packing in the column[57] |
(甲基)丙烯酸酯是最为常见的自由基光聚合单体.尽管许多室温下为固态的自由基光聚合单体均可以通过高能射线(如γ射线)引发聚合,从而获得满意的转化率,然而紫外光引发的固态光聚合一直少有报道.已有的关于自由基引发的固态光聚合均难以获得明显的转化率[4, 10, 59].这一方面是由于固态条件下分子运动能力高度受限,更为重要的是由于结晶态单体与无定型态的高聚物在结构上存在巨大差异,聚合会伴随着已有单体有序堆积结构的破坏,因此,单体在固态条件下的堆积方式阻碍了链增长反应的顺利进行.事实上,(甲基)丙烯酸酯单体的堆积方式,对于固态光聚合反应能否顺利进行起着决定性的作用.近些年来,Berchtold等[59]采用了如图5所示的两种类型的环缩醛聚氨酯丙烯酸酯单体(五元环和六元环),实现了自由基引发的固态光聚合.不同于双炔和二烯体系十分缓慢的固态光聚合,这类丙烯酸酯的光聚合具有快速的特点,聚合时间以秒计.他们进一步采用实时红外法监测了单体的结晶过程和光聚合过程,通过往复移动盐片,施加剪切力作用于夹在盐片中的液体来获得结晶态的单体.如图5所示,在相同的温度下(如图所示聚合温度为25 ℃),由剪切获得的结晶单体的固态光聚合具有比同样温度下的液态单体更高的聚合速率和双键转化率,Berchtold等认为这是由于单体的堆积方式有利于链增长反应的进行.
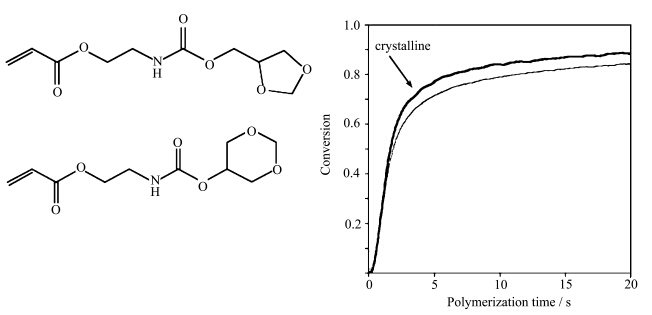 | 图5 环缩醛聚氨酯丙烯酸酯的化学结构(光聚合单体组成包含27%五元环环缩醛聚氨酯丙烯酸酯 和73%六元环环缩醛聚氨酯丙烯酸酯(左侧))与25 ℃下的固态光聚合和液态光聚合的对比(右侧)[59] Chemical structures of cyclic acetal urethane acrylate where the monomer polymerized here contains approximately 27% 5-membered ring acetal and 73% 6-membered acetal(left)and conversion vs time of cyclic acetal urethane acrylate polymerization of a monomer with ~27% 5-membered acetals at 25 ℃ Crystalline and noncrystalline state polymerizations are compared at same temperature(right)[59] |
在最近的研究中,蹇钰等[60,61]利用甲基丙烯酸十八酯和丙烯酸十八酯体系实现了快速的自由基引发的固态光聚合.这类长链烷烃的丙烯酸酯单体的特殊之处在于可以形成侧链结晶的聚合物.值得注意的是聚合物长侧链的堆积方式与单体α晶型的堆积方式一致,均为六方堆积.丙烯酸十八酯和甲基丙烯酸十八酯α晶型的熔点分别为30 ℃和20 ℃,因此,均可以十分方便的通过冷却熔融液体来得到α晶型.同时,为了避免汞灯的热量产生影响,聚合过程采用了冷光源的LED,并减小了涂布厚度(约13 μm),有效避免了热量对聚合过程中单体结晶状态造成的影响.通过实时红外法监测聚合过程,发现与结晶长链堆积方式相关的红外吸收峰均未发生变化,表明聚合过程并没有发生晶型的破坏,单体的六方堆积的长链结构被保留下来,形成聚合物中六方堆积的长侧链结构.由于这种单体和聚合物堆积方式一致,六方堆积单体有助于链增长的进行,同时也抑制了链终止,聚合反应通过最小的基团运动就可以完成.对于甲基丙烯酸十八酯(α晶型的熔点为20 ℃)的固态光聚合研究[60]表明,聚合速率和转化率均高于更高温度下的液态甲基丙烯酸十八酯的聚合(图6);固态光聚合制备的聚合物分子量更高,而多分散性更低(表2),表明此方法很好的克服了甲基丙烯酸酯体系因甲基的空间位阻效应和推电子基效应造成的聚合速率低的问题.
 | 图6 甲基丙烯酸酯在不同温度下固态光聚合与液态光聚合的对比 [TPO]= 1%(质量分数); 光强 5 mW/cm2,385 nm (LED) [60] Effect of temperature on the polymerization of octadecyl methacrylate [TPO]= 1%(mass fraction); light intensity 5 mW/cm2,385 nm (LED) [60] |
| 表2 不同温度下固态光聚合与液态光聚合对聚合物分子量和分子量分布的影响 [TPO]= 1 %(质量分数),光强 5 mW/cm2,385 nm (LED) [60] Effect of temperature on molecular weight and molecular weight distributions [TPO]= 1%(mass fraction),light intensity 5 mW/cm2,385 nm (LED) [60] |
这类固态单体的应用优势在于克服了液态自由基光聚合所固有的问题(包括氧阻聚和体积收缩).研究表明[61],由于结晶单体抑制了氧气的进入,因此相较于氧气阻聚十分显著的液态丙烯酸酯体系,丙烯酸十八酯的固态光聚合具有很好的抗氧阻聚的性能,转化率并不会因为接触氧气而明显的降低.同时由于结晶过程伴随着密度的提高,拉近了双键的距离,甲基丙烯酸十八酯和丙烯酸十八酯的固态光聚合均伴随着极低的体积收缩率,只有1%[61,①].
对于两官能的丙烯酸酯单体的固态光聚合的研究工作也得到了开展[62,②].吕波等[63]研究了三甘醇二甲基丙烯酸酯在低温条件下的光聚合,用比重瓶法测量了体积收缩,结 果发现聚合温度越低,体积的收缩越少,甚至出现体积膨胀现象.在常温条件下体积收缩为12.9%,-60 ℃时为2.4%,温度降低到-70 ℃时,没有发生体积收缩,甚至出现体积膨胀,为-1.6%.这是因为光聚合在低温下发生,而生成的高分子膜放置于室温后,随着温度的上升出现体积膨胀,从而弥补聚合所造成的收缩.季丽丽等人研究了常用的两官能丙烯酸酯单体(HDDA)的固态光聚合,首次制备了HDDA的单晶,单晶XRD衍射的结果表明HDDA单体的堆积方式为三斜堆积,如图7所示.
 | 图7 HDDA晶体(左侧)和晶体结构(右侧) The digital photo of HDDA crystal (left) and crystal structure of HDDA (right) |
研究表明[62]HDDA在低于其熔点温度(-10 ℃)的固态条件下,仍可具有一定的双键转化率(DC),如表3所示.HDDA的聚合活性随着温度的降低而明显降低,但体积收缩率(VS)的降低更为显著.因此在比较理论收缩率(即100 %双键转化条件下的收速率,由VS/DC计算得到)时,发现低温条件下具有更低的理论收缩率.
| 表3 不同温度下HDDA单体的聚合转化率,收缩率和理论收缩率[62] Degree of conversion,calculated and experimental shrinkage for HDDA under different temperatures[62] |
除了上述三种报道较多的固态光聚合体系,研究者们也在其他体系的固态光聚合研究方面取得了进展.Soldatov等[64]报道了采用固态光聚合的方法合成C70对应聚合物晶体的方法.对于C70单体而言,自由基聚合通常获得的是二聚体,无法获得C70的聚合物.Soldatov等发现在2 GPa压力和300 ℃的温度的条件下六方堆积的C70晶体可以在光照下聚合.固态核磁和拉曼光谱表明C70间形成了共价键;单晶XRD衍射表明形成了聚合物的晶体.固态光聚合也被报道用于合成二维结构的聚合物.通常认为,石墨烯是一种典型的二维结构的原子晶体,为了合成类似于石墨烯的二维层状结构,Kissel等[65]设计了如图8所示的单体,这种单体可以形成六方堆积的单晶.在氩气的保护下,单晶可由300 mW、470 nm的可见光引发聚合.TEM、AFM和拉曼光谱表明单体的结晶结构被保留并成功形成类似于石墨烯的二维层状结构的聚合物.此外,姚伯龙等[66]将丙烯酰胺、水溶性聚合物、表面活性剂和光引发剂配成水溶液,将溶剂挥发后,丙烯酰胺在水溶性聚合物上结晶形成单体膜,实验表明诱导产生的丙烯酰胺的单体膜可以很好的在固态条件下发生光聚合.
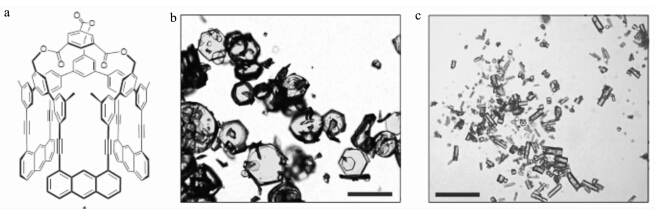 | 图8 二维预组装单体晶体:单体化学结构(a)和单晶的光学显微镜照片(b)和柱状(c) [67] Two-dimensional pre-organization in the monomer crystal:chemical structure of the monomer (a) and optical microscopy images of its single crystals obtained as plates (b) and rods (c) [67] |
无论在应用方面还是学术研究方面,固态光聚合都是光聚合技术发展的一个重要的领域.可以预见,随着研究的不断深入,将有更多的聚合体系实现高效、可控、环境友好的固态光聚合,从而推动光聚合技术的不断发展.
| [1] | Fisher J P, Dean D, Engel P S, et al. Photoinitiated polymerization of biomaterials [J]. Annual review of materials research, 2001, 31(1): 171-181. |
| [2] | Decker C. Photopolymerization and Ultraviolet Curing of Multifunctional Monomers [M]. Materials Science and Technology. Wiley-VCH Verlag GmbH & Co. KGaA. 2006. |
| [3] | Decker C. The use of UV irradiation in polymerization [J]. Polymer International, 1998, 45(2): 133-141. |
| [4] | Yagci Y, Jockusch S, Turro N J. Photoinitiated polymerization: advances, challenges, and opportunities [J]. Macromolecules, 2010, 43(15): 6245-6260. |
| [5] | Fouassier J P. Photoinitiation, Photopolymerization, and Photocuring: Fundamentals and Applications [M]. Hanser-Gardner Publications, 1995. |
| [6] | Decker C. Surface protection of poly(vinyl chloride) by photografting of epoxy-acrylate coatings [J]. Journal of Applied Polymer Science, 1983, 28(1): 97-107. |
| [7] | Kaczmarek H, Decker C. Interpenetrating polymer networks. I. Photopolymerization of multiacrylate systems [J]. Journal of Applied Polymer Science, 1994, 54(13): 2147-2156. |
| [8] | Moussa K, Decker C. Semi-interpenetrating polymer networks synthesis by photocrosslinking of acrylic monomers in a polymer matrix [J]. Journal of Polymer Science Part A: Polymer Chemistry, 2003, 31(10): 2633-2642. |
| [9] | Schnabel W. Polymers and Light: Fundamentals and Technical Applications [M]. Wiley-VCH Weinheim, Germany, 2007. |
| [10] | Satoh K, Kamigaito M. Stereospecific living radical polymerization: dual control of chain length and tacticity for precision polymer synthesis [J]. Chemical Reviews, 2009, 109(11): 5120-5156. |
| [11] | Baughman R. Solid-state synthesis of large polymer single crystals [J]. Journal of Polymer Science: Polymer Physics Edition, 1974, 12(8): 1511-1535. |
| [12] | Wegner G. Solid-state polymerization mechanisms [J]. Pure and Applied Chemistry, 1977, 49: 443-454. |
| [13] | Nakanishi H, Jones W, Thomas J, et al. Topochemically controlled solid-state polymerization [J]. Proceedings of the Royal Society of London A Mathematical and Physical Sciences, 1980, 369(1738): 307-325. |
| [14] | Bässler H. Photopolymerization of Diacetylenes. In: Polydiacetylenes[M]. Advances in Polymer Science, Volume 63. Springer,1984. 1-48. |
| [15] | Enkelmann V. Structural Aspects of the Topochemical Polymerization of Diacetylenes. In: Polydiacetylenes[M]. Advances in Polymer Science, Volume 63. Springer,1984. 91-136. |
| [16] | Baughman R. Solid-state polymerization of diacetylenes [J]. Journal of Applied Physics, 1972, 43(11): 4362-4370. |
| [17] | Chance R, Patel G. Solid-state polymerization of a diacetylene crystal: thermal, ultraviolet, and γ-ray polymerization of 2, 4-hexadiyne-1, 6-diol bis-(p-toluene sulfonate) [J]. Journal of Polymer Science: Polymer Physics Edition, 1978, 16(5): 859-881. |
| [18] | Enkelmann V, Leyrer R, Wegner G. Investigation of the topochemical solid-state polymerization of a diacetylene by X-ray methods and brillouin-spectroscopy [J]. Die Makromolekulare Chemie, 1979, 180(7): 1787-1795. |
| [19] | Patel G, Chance R, Turi E, et al. Energetics and mechanism of the solid-state polymerization of diacetylenes [J]. Journal of the American Chemical Society, 1978, 100(21): 6644-6649. |
| [20] | Matsuda H, Nakanishi H, Hosomi T, et al. Synthesis and solid-state polymerization of a new diacetylene: 1-(N-carbazolyl) penta-1, 3-diyn-5-ol [J]. Macromolecules, 1988, 21(5): 1238-1240. |
| [21] | Melveger A, Baughman R. Raman spectral changes during the solid-state polymerization of diacetylenes [J]. Journal of Polymer Science: Polymer Physics Edition, 1973, 11(4): 603-619. |
| [22] | Sixl H. Spectroscopy of the Intermediate States of the Solid State Polymerization Reaction in Diacetylene Crystals. In: Polydiacetylenes[M]. Advances in Polymer Science, Volume 63. Springer,1984. 49-90. |
| [23] | Iida R, Kamatani H, Kasai H, et al. Solid-state polymerization of diacetylene microcrystals [J]. Molecular Crystals and Liquid Crystals, 1995, 267(1): 95-100. |
| [24] | Wegner G. Topochemical polymerization of monomers with conjugated triple bonds [J]. Die Makromolekulare Chemie, 1972, 154(1): 35-48. |
| [25] | Matsuda H, Nakanishi H, Minami N, et al. Synthesis and solid-state polymerization of novel diacetylenes for nonlinear optics [J]. Molecular Crystals and Liquid Crystals, 1988, 160(1): 241-251. |
| [26] | Enkelmann V, Wenz G, Müller M, et al. Solved and unsolved problems in the solid-state polymerization of diacetylenes [J]. Molecular Crystals and Liquid Crystals, 1984, 105(1): 11-39. |
| [27] | Yee K. Synthesis and thermal solid-state polymerization of a new diacetylene: 2, 4-hexadiynylene bis (p-fluorobenzenesulfonate) [J]. The Journal of Organic Chemistry, 1979, 44(14): 2571-2573. |
| [28] | Wang Y, Li L, Yang K, et al. Nanocrystalline TiO2-catalyzed solid-state polymerization of diacetylene in the visible region [J]. Journal of the American Chemical Society, 2007, 129(23): 7238-7239. |
| [29] | Tieke B, Bloor D. Raman spectroscopic studies of the solid-state polymerization of diacetylenes, 3. UV-polymerization of diacetylene langmuir-blodgett multilayers [J]. Die Makromolekulare Chemie, 1979, 180(9): 2275-2278. |
| [30] | Okawa Y, Aono M. Materials science: nanoscale control of chain polymerization [J]. Nature, 2001, 409(6821): 683-684. |
| [31] | Kim J M, Ji E K, Woo S, et al. Immobilized polydiacetylene vesicles on solid substrates for use as chemosensors [J]. Advanced Materials, 2003, 15(13): 1118-1121. |
| [32] | Jahnke E, Lieberwirth I, Severin N, et al. Topochemical polymerization in supramolecular polymers of oligopeptide-functionalized diacetylenes [J]. Angewandte Chemie International Edition, 2006, 45(32): 5383-5386. |
| [33] | Ouyang X, Fowler F W, Lauher J W. Single-crystal-to-single-crystal topochemical polymerizations of a terminal diacetylene: two remarkable transformations give the same conjugated polymer [J]. Journal of the American Chemical Society, 2003, 125(41): 12400-12401. |
| [34] | Papaspyrides C D, Vouyiouka S N, MyiLibrary. Solid State Polymerization [M]. Wiley Online Library, 2009. |
| [35] | Hasegawa M, Suzuki Y, Suzuki F, et al. Four-center type photopolymerization in the solid state. I. Polymerization of 2, 5-distyrylpyrazine and related compounds [J]. Journal of Polymer Science Part A-1: Polymer Chemistry, 1969, 7(2): 743-752. |
| [36] | Nakanishi H, Suzuki Y, Suzuki F, et al. Four-center type photopolymerization in the solid state. II. Polymerization mechanism of 2, 5-distyrylpyrazine [J]. Journal of Polymer Science Part A-1: Polymer Chemistry, 1969, 7(2): 753-766. |
| [37] | Suzuki F, Suzuki Y, Nakanishi H, et al. Four-center type photopolymerization in the solid state. III. Polymerization of phenylene diacrylic acid and its derivatives [J]. Journal of Polymer Science Part A-1: Polymer Chemistry, 1969, 7(8): 2319-2331. |
| [38] | Nakanishi F, Hasegawa M. Four-center type photopolymerization in the solid state. IV. Polymerization of α, α'-dicyano-p-benzenediacrylic acid its derivatives [J]. Journal of Polymer Science Part A-1: Polymer Chemistry, 1970, 8(8): 2151-2160. |
| [39] | Nakanishi H, Hasegawa M, Sasada Y. Four-center type photopolymerization in the crystalline state. V. X-ray crystallographic study of the polymerization of 2, 5-distyrylpyrazine [J]. Journal of Polymer Science Part A-2: Polymer Physics, 1972, 10(8): 1537-1553. |
| [40] | Nakanishi H, Nakanishi F, Suzuki Y, et al. Four-center type photopolymerization in the crystalline state. VI. Kinetic and structural study of the polymerization of p-phenylenediacrylic acid diethyl ester [J]. Journal of Polymer Science: Polymer Chemistry Edition, 1973, 11(10): 2501-2517. |
| [41] | Nakanishi F, Nakanishi H, Hasegawa M, et al. Four-center type photopolymerization in the solid state. VII. Photochemical reaction of m-phenylene diacrylic acid dimethyl ester [J]. Journal of Polymer Science: Polymer Chemistry Edition, 1975, 13(11): 2499-2506. |
| [42] | Meyer W, Lieser G, Wegner G. Four-center photopolymerization. A heterogeneous topochemical solid-state reaction [J]. Journal of Polymer Science: Polymer Physics Edition, 1978, 16(8): 1365-1379. |
| [43] | Hasegawa M. Four-center photopolymerization in the crystalline state. In: New Polymerization Reactions [M]. Springer, 1982. 1-49. |
| [44] | Hasegawa M. Photopolymerization of diolefin crystals [J]. Chemical Reviews, 1983, 83(5): 507-518. |
| [45] | Matsumoto A, Matsumura T, Aoki S. Stereospecific polymerization of dialkyl muconates through free radical polymerization: isotropic polymerization and topochemical polymerization [J]. Macromolecules, 1996, 29(1): 423-432. |
| [46] | Odani T, Matsumoto A, Sada K, et al. One-way EZ-isomerization of bis (n-butylammonium)(Z, Z)-muconate under photoirradiation in the crystalline state [J]. Chemical Communications, 2001, (19): 2004-2005. |
| [47] | Matsumoto A, Furukawa D, Nakazawa H. Stereocontrol of diene polymers by topochemical polymerization of substituted benzyl muconates and their crystallization properties [J]. Journal of Polymer Science Part A: Polymer Chemistry, 2006, 44(17): 4952-4965. |
| [48] | Matsumoto A, Oshita S, Fujioka D. A novel organic intercalation system with layered polymer crystals as the host compounds derived from 1,3-diene carboxylic acids [J]. Journal of the American Chemical Society, 2002, 124(46): 13749-13756. |
| [49] | Matsumoto A, Odani T, Sada K, et al. Intercalation of alkylamines into an organic polymer crystal [J]. Nature, 2000, 405(6784): 328-330. |
| [50] | Nakamoto S, Tashiro K, Matsumoto A. Vibrational spectroscopic study on the molecular deformation mechanism of a poly(trans-1,4-diethyl muconate) single crystal subjected to tensile stress [J]. Macromolecules, 2003, 36(1): 109-117. |
| [51] | Furukawa D, Kobatake S, Matsumoto A. Direct observation of change in the molecular structure of benzyl (Z, Z)-muconate during photoisomerization in the solid state [J]. Chemical Communications, 2008, (1): 55-57. |
| [52] | Nagahama S, Tanaka T, Matsumoto A. Supramolecular control over the stereochemistry of diene polymers [J]. Angewandte Chemie International Edition, 2004, 43(29): 3811-3814. |
| [53] | Matsumoto A, Matsumura T, Aoki S. Stereospecific polymerisation of diethyl (Z, Z)-hexa-2, 4-dienedioate in the crystalline state [J]. Journal of the Chemical Society, Chemical Communications, 1994, (11): 1389-1390. |
| [54] | Matsumoto A, Tanaka T, Tsubouchi T, et al. Crystal engineering for topochemical polymerization of muconic esters using halogen-halogen and CH/π interactions as weak intermolecular interactions [J]. Journal of the American Chemical Society, 2002, 124(30): 8891-8902. |
| [55] | Matsumoto A, Yokoi K, Aoki S, et al. Crystalline-state polymerization of diethyl (Z, Z)-2, 4-hexadienedioate via a radical chain reaction mechanism to yield an ultrahigh-molecular-weight and stereoregular polymer [J]. Macromolecules, 1998, 31(7): 2129-2136. |
| [56] | Matsumoto A, Yokoi K. Control of molecular weight of the polymers produced during the crystalline-state photopolymerization of diethyl cis, cis-muconate as studied by gel permeation chromatography and scanning electron micrography [J]. Journal of Polymer Science Part A: Polymer Chemistry, 1998, 36(17): 3147-3155. |
| [57] | Matsumoto A, Nagahama S, Odani T. Molecular design and polymer structure control based on polymer crystal engineering. Topochemical polymerization of 1, 3-diene mono-and dicarboxylic acid derivatives bearing a naphthylmethylammonium group as the countercation [J]. Journal of the American Chemical Society, 2000, 122(38): 9109-9119. |
| [58] | Matsumoto A, Tanaka T, Tsubouchi T, et al. Crystal engineering for topochemical polymerization of muconic esters using halogen-halogen and CH/π interactions as weak intermolecular interactions [J]. Journal of the American Chemical Society, 2002, 124(30): 8891-8902. |
| [59] | Berchtold K A, Hacioglu B, Nie J, et al. Rapid solid-state photopolymerization of cyclic acetal-containing acrylates [J]. Macromolecules, 2009, 42(7): 2433-2437. |
| [60] | Jian Y, He Y, Wang J, et al. Rapid photopolymerization of octadecyl methacrylate in the solid state [J]. New Journal of Chemistry, 2013, 37(2): 444-450. |
| [61] | Jian Y, He Y, Wang J, et al. Rapid solid-state photopolymerization of octadecyl acrylate: low shrinkage and insensitivity to oxygen [J]. Polymer International, 2013, doi: 10.1002/pi.4468. |
| [62] | Ji L L, Chang W K, Cui M, et al. Photopolymerization kinetics and volume shrinkage of 1,6-hexanediol diacrylate at different temperature [J]. Journal of Photochemistry and Photobiology a-Chemistry, 2013, 252: 216-221. |
| [63] | Lu B, Xiao P, Sun M, et al. Reducing volume shrinkage by low-temperature photopolymerization [J]. Journal of Applied Polymer Science, 2007, 104(2): 1126-1130. |
| [64] | Soldatov A V, Roth G, Dzyabchenko A, et al. Topochemical polymerization of C70 controlled by monomer crystal packing [J]. Science, 2001, 293(5530): 680. |
| [65] | Kissel P, Erni R, Schweizer W B, et al. A two-dimensional polymer prepared by organic synthesis [J]. Nature Chemistry, 2012, 4(4): 287-291. |
| [66] | 姚伯龙,张小玲. 固态晶相光聚合新技术 [J]. 无锡轻工大学学报, 2001, 20(6): 14. Yao B L, Zhang X L. Study on a new type of solid-state crystalline photopolymerization[J]. Journal of Wuxi University of Light Industry, 2001, 20(6): 14. |