2. 中国科学院 化学研究所 光化学院重点实验室, 北京 100190
2. Key Labaratory of Photochemistry, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, P. R. China
聚合物微胶囊在药物封装和释放[1]、人造细胞[2]、催化[3]、化学传感器[4]等领域具有广阔的应用前景,因而引起了国内外研究学者的广泛关注。微胶囊的制备方法主要有乳化聚合法[5, 6]、膜乳化法[7]、层层自组装法[8]、微流体法[9]等。利用不同的制备方法可以得到不同尺寸、比表面积、形态、囊壁厚度的微胶囊,从而影响微胶囊对芯材物质的封装和释放行为。芯材物质可以经由囊壁上的孔隙向外扩散或通过破裂微胶囊达到释放的目的,当这种释放行为可控时,对于控制芯材物质作用效率、延长作用时间具有重要意义。
关于微胶囊的制备方法已有许多文献详细报道[10,11,12],本文将对近年来关于不同刺激响应型聚合物微胶囊的释放的研究进展进行综述。微胶囊的响应释放是由于外部环境的刺激引发囊壁材料渗透性的改变或是囊壁物质被破坏,使微胶囊芯材物质由内向外的扩散过程发生改变。这些外部环境的改变大体可以分成两大类:化学变化和物理变化。化学变化,是指微胶囊的囊壁材料与外界环境发生化学反应,改变囊壁材料的化学性质;物理变化,是指微胶囊在外部环境的作用下发生相转换,或外部对其施加机械力等,是一种不改变囊壁材料的化学性质,只改变其连续性或完整性的方式。 2 不同刺激响应型微胶囊的可控释放的研究进展 2.1 温敏型微胶囊
温度的变化能引起微胶囊/聚合物微球的熔化或者导致相转换[13]、体积膨胀[14]或含水量减少从而导致胶囊萎缩[15, 16]。除了将材料直接加热之外,有些由外加磁场、光、化学物质、电刺激等引发微胶囊释放的根本原因也是由于体系温度的变化。本节只介绍直接变化外部温度调控微胶囊释放的模式。
温敏型微胶囊通常采用温敏聚合物材料作为囊壁,温度变化会引起聚合物发生相变,在囊壁上产生毛细孔,导致芯材物质的流出。此类聚合物以聚N-异丙基丙烯酰胺(PNIPAm)为代表,当温度升高至PNIPAm的最低临界溶解温度(LCST)时,聚合物由于相变发生收缩,从而在囊壁上产生小孔,囊壁渗透性增强,触发释放[17, 18]。
PNIPAm相转换温度大约在人体体温范围内,因此此类微胶囊常应用于药物传输、仿生材料等领域,但由于不能在生物条件下被降解,使其应用有一定的局限性。
另一类温敏型微胶囊的释放是由温度升高引起囊壁物质的解组装进而触发芯材物质释放。Lutkenhaus等将聚二甲基二烯丙基氯化铵(PDAC)、聚苯乙烯磺酸钠(PSS)层层自组装,以地塞米松(DXM)为核制备了一种温敏型微胶囊[19]。通过研究聚电解质层数、离子强度、温度等因素对微胶囊释放过程的影响,作者得出结论:微胶囊的释放是由于温度的升高引起了微胶囊囊壁材料的膨胀,聚电解质解组装,从而增大了层与层之间的空隙,使芯材物质扩散出来(如图1所示[19])。
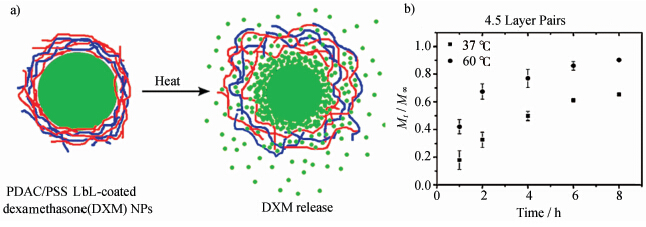 | 图1 a) 温敏型聚电解质微胶囊内芯材物质的释放过程示意图,b) 在37 ℃和60 ℃的条件下DXM的累计释放曲线 a) Schematic representation of the release of DXM from thermo-responsive PDAC/PSS polyelectrolyte microcapsules, b) cumulative release of DXM at 37 ℃ (square) and 60 ℃ (circle) |
近年来,通过改变微胶囊内部或外部环境的pH值来改变囊壁材料的性质或结构,引发微胶囊释放,已经成为一种很普遍的控制微胶囊释放的方式[20,21,22]。
其中一种是通过改变酸碱性环境,使囊壁材料发生共价键的变化。比如Fréchet课题组采用缩酮作为囊壁材料,当pH小于5时,每个缩酮降解转换成一个酮和两个醇,囊壁材料的化学降解使微胶囊外壳破裂,使得微胶囊在1 h内将50%的芯材物质释放出来[23]。
另一种是通过调节质子的加入量来破坏离子键实现开关式释放。Caruso等用层层自组装的方法合成了不同聚电解质组成的纳米多孔聚合物微球,包括聚丙烯酸树脂(PAA)、聚丙烯酰胺(PAAm)、PSS等[24]。结果表明,pH值降低使聚合物之间的静电吸引作用减弱,同时使聚合物微球与内部装载的蛋白质同带正电荷,产生静电排斥作用,从而将90%的蛋白质在1 h内释放出来,并且在pH为7的条件下清洗,可以恢复聚合物微球对蛋白质的装载能力。
以氢键结合的囊壁材料同样可以实现pH响应,例如Tsukruk课题组通过聚合物层层自组装的方法用丹宁酸(TA)组装成由氢键结合的囊壁,微胶囊在pH值为2至10的范围内保持稳定[25]。当pH值在这一范围之外时,囊壁TA分子发生氢键断裂引起芯材物质的释放。
pH响应型微胶囊的可控释放在癌症化疗领域受到了极大的关注,主要是因为肿瘤组织微环境的pH值通常比健康的组织的pH值低0.5~1.0[26],引入pH响应型材料,通过调节微胶囊的组分、囊壁厚度,以及pH值便可调控药物的释放速率和有效作用时间。 2.3 磁响应型微胶囊
为了实现微胶囊的靶向传输以及可控释放,许多研究者在微胶囊的囊壁[27,28,29]或内部[30, 31]引入磁性纳米颗粒。目前,磁响应微胶囊的释放主要有两种模式:
一种是通过高频交变磁场下磁性颗粒的磁热效应[32, 33],诱导温敏型囊壁材料发生变化,实现可控释放。例如:刘典谟课题组将Fe3O4磁性纳米颗粒嵌入PAA-PSS微胶囊囊壁中,在交变磁场的作用下温度升高使芯材物质释放出来[33]。在这种情况下,无论是从显微镜观察还是从释放曲线都可以看出,释放过程开始时缓慢,而后随着微胶囊的破裂迅速加快。作者认为这是由于交变磁场作用下温度升高,引起囊壁材料的结构变化使微胶囊破裂(如图2所示[33])。
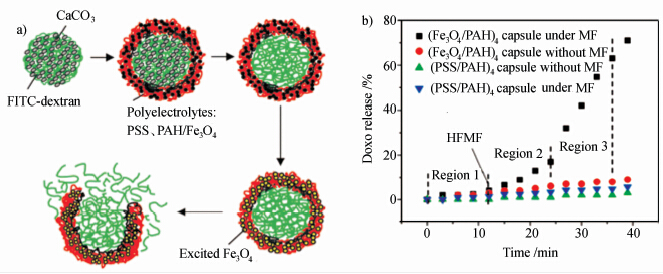 | 图2 a)磁响应聚合物微胶囊内芯材物质的封装及释放过程的示意图,b) 高频磁场作用下(Fe3O4/PAH)4胶囊的药物释放曲线 a) Schematic representation of the encapsulation and release of magnetic responsive polyelectrolyte microcapsules, b) drug release profiles of (Fe3O4/PAH)4 and (PSS/PAH)4 microcapsules with and without a magnetic field |
另一种则是利用高频交变磁场下囊壁材料中磁性颗粒的快速旋转摆动,用机械力破坏微胶囊囊壁的完整性,从而达到可控释放的目的。例如:Kumar和Lvov将磁性的Co@Au纳米颗粒嵌入PAH-PSS的囊壁中[27],结果表明在交变磁场的作用下,Co@Au纳米颗粒发生快速旋转,从而破坏了多层膜的结构,增强了微胶囊囊壁的渗透性。
磁响应微胶囊的最大优势在于可以实现靶向传输,与其他传输方式相比其治疗效果十分显著,而且相比其他调控手段,磁场变化对生物体环境影响也最小。但目前的磁控手段通常伴随着温度变化或机械运动,因此,如何减少这些干扰,成为了目前磁响应微胶囊研究过程中的难点。 2.4 电响应型微胶囊
电响应型微胶囊在电子墨水显示技术[34]、耐腐蚀[35, 36]、电子自我修复[37, 38]以及药物传输[35]等方面得到了广泛的应用。大量的电响应材料被用作微胶囊的囊壁材料。在外加电场作用下,根据囊壁材料的不同,通过导电过程使囊壁材料分子排布发生变化,实现微胶囊的可控释放。
例如Kim等报道了一项在电场作用下提高微胶囊释放速率的研究工作[39]。他们制备了一种由聚乙烯醇(PVA)、聚丙烯酸(PAAc)和多壁碳纳米管(MWCNT)组成囊壁的复合微胶囊,研究表明均匀分布的碳纳米管形成了具有良好导电性的网状结构,在电场的作用下,碳纳米管导电加快了聚合物长链上羧基的电离,随电离度升高,聚合物间静电排斥作用逐渐增强,最终引起微胶囊的膨胀和芯材的释放。芯材物质的释放速率随外部电压的升高和碳纳米管的有效分散而增加(如图3所示[39])。
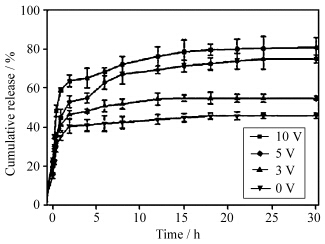 | 图3 MWCNT/PVA/PAAc微胶囊在不同电压的电场作用下的累积释放曲线 Cumulative release of MWCNT/PVA/PAAc microcapsules at various voltages |
利用酶、糖类、核苷酸序列等广泛应用于生命体系中的生物分子之间的相互作用也可以实现微胶囊的可控释放。Glangchai等利用组织蛋白酶B将GFLGK缩氨酸交叉链分解从而引发纳米粒子将内部的DNA分子释放出来[40]。唐义等[41]通过界面聚合的方法将目标蛋白质封装在纳米微胶囊中,并将一种可以被蛋白酶水解的多肽与微胶囊交联起来,一旦发生蛋白水解作用,内部的蛋白质便释放出来。这种由蛋白酶引发的释放过程可以通过改变与多肽交联的光敏性保护基团,从时间和空间方面进行控制。酯酶作为一种分解酯类的酶,通过改变微胶囊的亲油性也被用于分解一种由树枝状分子构成的纳米微胶囊[42]。
这种生物触发方式具有更好的生物体系相容性,但其缺点是很难精确的控制微胶囊释放的初始时间。 2.6 光响应型微胶囊
目前,通过光照对微胶囊释放芯材物质进行调控的模式引起了许多研究人员的关注。将光敏性纳米粒子或化合物分子嵌入在囊壁材料中,吸收某一波段的光之后,利用光热效应或光化学反应,引起囊壁材料变化,调控芯材物质释放[43,44,45,46]。微胶囊的光响应方式一般为利用金属纳米粒子的光热效应使囊壁聚电解质变化,或利用光催化、光诱导等引发光异构化、光分解或光加成反应,使囊壁材料发生变化,最终实现光响应控制释放。
金属纳米颗粒广泛存在于光引发微胶囊释放的反应中。Caruso等发现囊壁带有金纳米颗粒的聚电解质微胶囊在激光照射下可以发生响应性释放[47]。作者指出,激光响应释放过程分为以下三步:首先,纳米粒子吸收光后发热使微胶囊囊壁物质温度升高至水的亚稳态温度;接下来,微胶囊内部的热应力随囊壁材料的热膨胀而发生变化;最终,微胶囊破裂。同样,Skirtach等也得到了一种由金的硫化物和金组成的核-壳结构的纳米颗粒,在激光照射下纳米颗粒升温使囊壁温度升高引起微胶囊的破裂[48]。West等将金纳米颗粒嵌入PNIPAm微胶囊囊壁上,在近红外光的照射下,随着温度的升高微胶囊的渗透性增强[49]。
利用金属氧化物涂层的光催化作用也可以被用作微胶囊光响应释放的触发器。Katagiri等得到了一种对紫外光有响应性的微胶囊,聚电解质层层自组装得到微胶囊,外层覆盖SiO2/TiO2涂层,TiO2吸收紫外光后光催化囊壁有机材料分解(如图4所示[50]),使微胶囊破裂,可通过调节SiO2和TiO2的比例控制微胶囊的释放速率[50]。
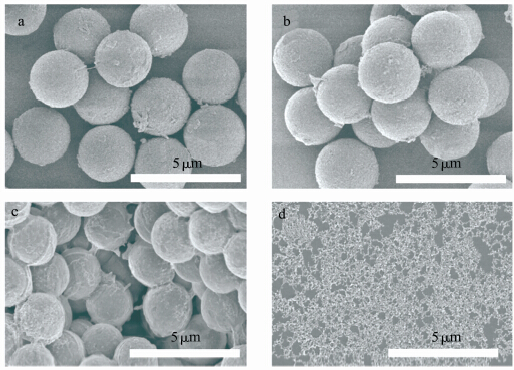 | 图4 a、b为(PSS/PDDA)5/PSS/DDAB/SiO2微胶囊的SEM照片;c、d为(PSS/PDDA)5/PSS/DDAB/SiO2-TiO2 (75∶25)微胶囊的SEM照片;a、c和b、d分别为微胶囊在20 mW/cm的紫外光下照射60 min前后的照片 SEM images of (a, b) (PSS/PDDA)5/PSS/DDAB/SiO2 capsules and (c, d) (PSS/PDDA)5/PSS/DDAB/SiO2-TiO2 (75∶25) capsules (a, c) before and (b, d) after UV irradiation (20 mW/cm, 60 min) |
偶氮苯之类的光异构化基团在紫外-可见光的作用下可以发生可逆的顺反异构反应,引发微胶囊释放,这是一类最早被引入光响应微胶囊释放的方式。Mhwald将一种偶氮类染料引入微胶囊的囊壁,结果发现在可见光的照射下微胶囊的渗透性发生了改变[51]。Bédard等同样证明了当偶氮染料连接在聚电解质的主链上时,偶氮苯部分吸收光后会改变聚电解质微胶囊的渗透性[52]。通过改变主链上偶氮染料的相对用量可以控制芯材物质的释放速率。
带有光敏基团的自分解聚合物也可以用来引发聚合物微胶囊的释放。Almutairi等报道了一种光敏性的自分解聚合物,这种聚合物带有光敏性苄酯基,是以带有两个甲氧基和一个硝基的苯环为侧链的聚合物[53]。作者观测到波长为350 nm的光照射可以引发微胶囊内部染料的释放,而没有光照时没有检测到染料释放,这一现象表明染料的释放是由于主链上的苄酯基在光诱导下发生自分解反应而引发的(如图5所示[53])。
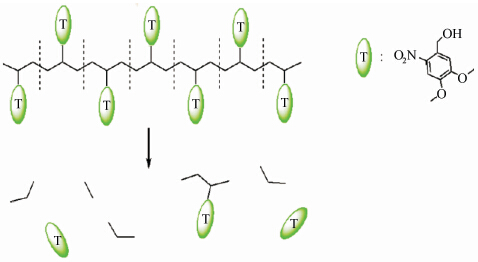 | 图5 光照下光敏性聚合物的分解 Degradation of light-sensitive polymer upon irradiation |
Schärt等采用了另外一种方法进行光引发微胶囊释放。他们用硝基肉桂酸对聚硅氧烷纳米颗粒进行功能化处理。在Hg/Xe灯照射下,两个肉桂酸分子发生[2+2]环加成反应,将纳米颗粒交联起来,形成微胶囊[54]。当另外选用250 nm波长的紫外光照射时,环加成产物又发生开环反应,导致微胶囊囊壁分解,达到光控释放的目的。
光响应微胶囊具有绿色环保、可实现远距离调控等优点。由于紫外光的普遍存在,紫外-可见光光敏型微胶囊被广泛应用于化妆品和农业生产领域[55, 56]。而在生物组织内应用的光敏型微胶囊多对近红外光响应,主要是因为近红外波长的光在生物组织中有良好的穿透性[57,58,59]。 3 总结与展望
本文大致归纳了目前刺激响应型微胶囊的主要种类,分别是:温敏型微胶囊、pH响应型微胶囊、磁响应型微胶囊、电响应型微胶囊、生物响应型微胶囊和光响应型微胶囊,并对各类微胶囊的释放过程进行了举例介绍。微胶囊可控释放目前已在化工、医药、化妆品、农药等领域受到越来越多的关注。未来的微胶囊发展趋势是成本更加低廉、生产规模更大、刺激触发方式更灵敏、选择性更高,且更加适用于生物体(如采用静态磁场、近红外照射等)、微胶囊囊壁分解产物更加环境友好,等等。目前也发现微胶囊载入特殊功能的芯材物质,在特定条件下芯材释放,材料可起到自修复的作用,对未来的新型材料、电子芯片等领域的发展也具有重要意义。
| [1] | Skirtach A G, Javier A M, Kreft O, Köhler K, Alberola A P, Möhwald H, Parak W J, Sukhorukov G B. Laser-induced release of encapsulated materials inside living cells[J]. Angewandte Chemie International Edition, 2006, 45(28): 4612-4617. |
| [2] | Peters R J R W, Louzao I, Hest J C M V. From polymeric nanoreactors to artificial organelles[J]. Chemical Science, 2012,3(2): 335-342. |
| [3] | Zhang Q, Tiefenbacher K. Hexameric resorcinarene capsule is a bronsted acid: investigation and application to synthesis and catalysis[J]. Journal of the American Chemical Society, 2013, 135(43): 16213-16219. |
| [4] | Amali A J, Awwad N H, Rana R K, Patra D. Nanoparticle assembled microcapsules for application as pH and ammonia sensor[J]. Analytica Chimica Acta, 2011, 708(1-2): 75-83. |
| [5] | Zou S W, Hu Y, Wang C Y. One-pot fabrication of rattle-like capsules with multicores by pickering-based polymerization with nanoparticle nucleation[J]. Macromolecular Rapid Communications, 2014, 35(16): 1414-1418. |
| [6] | Yin W S, Yates M Z. Effect of interfacial energy on the formation of polymer microcapsules by emulsification/free drying[J]. Langmuir, 2008, 24(3): 701-708. |
| [7] | Wei Y, Wang Y X, Wang L Y, Hao D X, Ma G H. Fabrication strategy for amphiphilic microcapsules with narrow size distribution by premix membrane emulsification[J]. Colloids and Surfaces B: Biointerfaces, 2011, 87(2): 399-408. |
| [8] | Volodkin D V, Petrov A I, Prevot M, Sukhorukov G B. Matrix Polyelectrolyte Microcapsules: new system for macromolecule encapsulation[J]. Langmuir, 2004, 20(8): 3398-3406. |
| [9] | Shum H C, Kim J W, Weitz D A. Microfluidic fabrication of monodisperse biocompatible and biodegradable polymersomes with controlled permeability[J]. Journal of the American Chemical Society, 2008, 130(29): 9543-9549. |
| [10] | Kwon O S, Jang J, Bae J. A review of fabrication methods and applications of novel tailored microcapsules[J]. Current Organic Chemistry, 2013, 17(1): 3-13. |
| [11] | 王安河, 白振宇, 崔 岳, 李峻柏.环境多响应性微胶囊的制备[J].东南大学学报(医学版), 2011, 30(1): 33-39. Wang A H, Bai Z Y, Cui Y, Li J B. Synthesis of environmental multi-responsive microcapsules[J]. Journal of Southeast University(Medical Science Edition), 2011, 30(1):33-39. |
| [12] | Cui J W, Koeverden M P, M llner M, Kempe K, Caruso F. Emerging methods for the fabrication of polymer capsules[J]. Advances in Colloid and Interface Science, 2014, 207(SI):14-31. |
| [13] | Liu L, Wang W, Ju X J, Xie R, Xie L Y. Smart thermo-triggered squirting capsules for nanoparticle delivery[J]. Soft Matter, 2010,6(16): 3759-3763. |
| [14] | Fundueanu G, Constantin M, Ascenzi P, Simionescu B C. An intelligent multicompartmental system based on thermo-sensitive starch microspheres for temperature-controlled release of drugs[J]. Biomedical Microdevices, 2010, 12(4): 693-704. |
| [15] | Čejková J, Hanuš J, Štěpánek F. Investigation of internal microstructure and thermo-responsive properties of composite PNIPAM/silica microcapsules[J]. Journal of Colloid and Interface Science, 2010, 346(2): 352-360. |
| [16] | Kim B, Lee H S, Kim J, Kim S H. Microfluidic fabrication of photo-responsive hydrogel capsules[J]. 2013, 49(18): 1865-1867. |
| [17] | Sankaranarayanan J, Mahmoud, E A, Kim G, Morachis J M, Almutairi A. Multiresponse strategies to modulate burst degradation and release from nanoparticles[J]. ACS Nano, 2010, 4(10): 5930-5936. |
| [18] | Wang X, Li G Q, Wei J, Guan W W. A novel method to control microcapsule release behavior via photo-crosslink polyurethane acrylate shells[J]. Journal of Applied Polymer Science, 2009, 113(2): 1008-1016. |
| [19] | Zhou J, Pishko M V, Lutkenhaus J L. Thermoresponsive layer-by-layer assemblies for nanoparticle-based drug delivery[J]. Langmuir, 2014, 30(20): 5903-5910. |
| [20] | Liang K, Such G K, Johnston A P R, Zhu Z Y, Ejima H, Richardson J J, Cui J W, Caruso F. Endocytic pH-triggered degradation of nanoengineered multilayer capsules[J]. Advanced Materials, 2014, 26(12): 1901-1905. |
| [21] | Mauser T, D jugnat C, Sukhorukov G B. Reversible pH-dependent properties of multilayer microcapsules made of weak polyelectrolytes[J]. Macromolecular Rapid Communications, 2004, 25(20): 1781-1785. |
| [22] | Sato K, Yoshida K, Takahashi S, Anzai J. pH- and sugar-sensitive layer-by-layer films and microcapsules for drug delivery[J]. Advanced Drug Delivery Reviews, 2011, 63(9): 809-821. |
| [23] | Broaders K E, Pastine S J, Grandhe S, Fr chet J M. J. Acid-degradable solid-walled microcapsules for pH-responsive burst-release drug delivery[J]. Chemical Communications, 2011, 47(2): 665-667. |
| [24] | Wang Y J, Caruso F. Template synthesis of stimuli-responsive nanoporous polymer-based spheres via sequential assembly[J]. Chemistry of Materials, 2006, 18(17): 4089-4100. |
| [25] | Kozlovskaya V, Kharlampieva E, Drachuk I, Cheng D, Tsukruk V V. Responsive microcapsule reactors based on hydrogen-bonded tannic acid layer-by-layer assemblies[J]. Soft Matter, 2010, 6(15): 3596-3608. |
| [26] | Pasut G, Veronese F M. Polymer drug conjugation, recent achievements and general strategies[J]. Progress in Polymer Science, 2007, 32(8-9): 933-961. |
| [27] | Lu Z H, Prouty M D, Guo Z, Golub V O, Kumar C S S R, Lvov Y M. Magnetic switch of permeability for polyelectrolyte microcapsules embedded with Co@Au nanoparticles[J]. Langmuir, 2005, 21(5): 2042-2050. |
| [28] | Caruso F, Spasova M, Susha A, Giersig M, Caruso R A. Magnetic nanocomposite particles and hollow spheres constructed by a sequential layering approach[J]. Chemistry of Materials, 2001, 13(1): 109-116. |
| [29] | Fang M, Grant P S, McShane M J, Sukhorukov G B, Golub V O, Lvov Y M. Magnetic bio/nanoreactor with multilayer shells of glucose oxidase and inorganic nanoparticles[J]. Langmuir, 2002, 18(16): 6338-6344. |
| [30] | Chen Y, Chen H R, Zeng D P, Tian Y B, Chen F, Feng J W, Shi J L. Core/shell structured hollow mesoporous nanocapsules: a potential platform for simultaneous cell imaging and anticancer drug delivery[J]. ACS Nano, 2010, 4(10):6001-6013. |
| [31] | Chen H Y, Sulejmanovic D, Moore T, Colvin D C, Qi B, Mefford O T, Gore J C, Alexis F, Hwu S J, Anker J N. Iron-loaded magnetic nanocapsules for pH-triggered drug release and MRI imaging[J]. Chemistry of Materials, 2014, 26(6): 2105-2112. |
| [32] | Katagiri K, Nakamura M, Koumoto K. Magnetoresponsive smart capsules formed with polyelectrolytes, lipid bilayers and magnetic nanoparticles[J]. ACS Applied Materials & interfaces, 2010, 2(3): 768-773. |
| [33] | Hu S H, Tsai C H, Liao C F, Liu D M, Chen S Y. Controlled rupture of magnetic polyelectrolyte microcapsules for drug delivery[J]. Langmuir, 2008, 24(20): 11811-11818. |
| [34] | Wang J P, Zhao X P, Guo H L, Zheng Q. Preparation and response behavior of blue electronic ink microcapsules[J]. Optical Materials, 2008, 30(8): 1268-1272. |
| [35] | Ma Y J, Dong W F, Hempenius M A, Möhwald H, Vancso G J. Redox-controlled molecular permeability of composite-wall microcapsules[J]. Nature Materials, 2006, 5(9):724-729. |
| [36] | Jafari A H, Hosseini S M A, Jamalizadeh E. Investigation of smart nanocapsules containing inhibitors for corrosion protection of copper[J]. Electrochimica Acta, 2010, 55(28): 9004-9009. |
| [37] | Caruso M M, Schelkopf S R, Jackson A C, Landry A M, Braun P V, Moore J S. Microcapsules containing suspensions of carbon nanotubes[J]. Journal of Materials Chemistry, 2009, 19(34): 6093-6096. |
| [38] | Odom S A, Caruso M M, Finke A D, Prokup A M, Ritchey J A, Leonard J H, White S R, Sottos N R, Moore J S. Restoration of conductivity with TTF-TCNQ charge-transfer salts[J]. Advanced Functional Materials, 2010, 20(11): 1721-1727. |
| [39] | Yun J, Im J S, Lee Y S, Bae T S, Lim Y M, Kim H I. pH and electro-responsive release behavior of MWCNT/PVA/PAAc composite microcapsules[J]. Colloids and Surfaces A, 2010, 368(1-3): 23-30. |
| [40] | Glangchai L C, Moore M C, Shi L, Roy K. Nanoimprint lithography based fabrication of shape-specific, enzymatically-triggered smart nanoparticles[J]. Journal of Controlled Release, 2008, 125(3):263-272. |
| [41] | Gu Z, Yan M, Hu B, Joo K I, Biswas A, Huang Y, Lu Y F, Wang P, Tang Y. Protein nanocapsule weaved with enzymatically degradable polymeric network[J]. Nano Letters, 2009, 9(12): 4533-4538. |
| [42] | Azagarsamy M A, Sokkalingam P, Thayumanavan S. Enzyme-triggered disassembly of dendrimer-based amphiphilic nanocontainers[J]. Journal of the American Chemical Society, 2009, 131(40): 14184-14185. |
| [43] | Viger M L, Sheng W Z, Dor K, Alhasan A H, Carling C J, Lux J, Lux C de G, Grossman M, Malinow R, Almutairi A. Near-infrared-induced heating of confined water in polymeric particles for efficient payload release[J]. ACS Nano, 2014, 8(5): 4815-4826. |
| [44] | Matsumura A, Tsuchiya K, Torigoe K, Sakai K, Sakai H, Abe M. Photochemical control of molecular assembly formation in a catanionic surfactant system[J]. Langmuir, 2011, 27(5):1610-1617. |
| [45] | Dube H, Ajami D, Rebek Jr. J. Photochemical control of reversible encapsulation[J]. Angewandte Chemie International Edition, 2010, 49(18): 3192-3195. |
| [46] | Berryman O B, Sather A C, Rebek Jr J. A light controlled cavitand wall regulates guest binding[J]. Chemical Communications, 2011, 47(2): 656-658. |
| [47] | Angelatos A S, Radt B, Caruso F. Light-responsive polyelectrolyte/gold nanoparticle microcapsules[J]. Journal of Physical Chemistry B, 2005, 109(7): 3071-3076. |
| [48] | Skirtach A G, Dejugnat C, Braun D, Susha A S, Rogach A L, Wolfgang J P, Helmuth M, Gleb B S. The role of metal nanoparticles in remote release of encapsulated materials[J]. Nano Letters, 2005, 5(7): 1371-1377. |
| [49] | Sershen S R, Westcott S L, Halas N J, West J L. Temperature-sensitive polymer nanoshell composites for photothermally modulated drug delivery[J]. Journal of Biomedical Materials Research, 2000, 51(3): 293-298. |
| [50] | Katagiri K, Koumoto K, Iseya S, Sakai M, Matsuda A, Caruso F. Tunable UV-responsive organic-inorganic hybrid capsules[J]. Chemistry of Materials, 2009, 21(2): 195-197. |
| [51] | Tao X,Li J B, M hwald H.Self-assembly,optical behavior, and permeability of a novel capsule based on an azo dye and polyelectrolytes[J]. Chemistry-A European Journal, 2004, 10(14): 3397-3403. |
| [52] | Bédard M F, Geest B G D, Skirtach A G, M hwald H, Sukhorukov G B. Polymeric microcapsules with light responsive properties for encapsulation and release[J]. Advances in Colloid and Interface Science, 2010, 158(1-2): 2-14. |
| [53] | Fomina N, McFearin C, Sermsakdi M, Edigin O, Almutairi A. UV and near-IR triggered release from polymeric nanoparticles[J]. Journal of the American Chemical Society, 2010, 132(28): 9540-9542. |
| [54] | Yuan X F, Fischer K, Schärt W. Photocleavable microcapsules built from photoreactive nanospheres[J]. Langmuir, 2005, 21(20):9374-9380. |
| [55] | Yi Q Y, Sukhorukov G B. UV light stimulated encapsulation and release by polyelectrolyte microcapsules[J]. Advances in Colloid and Interface Science, 2014, 207(S1): 280-289. |
| [56] | Achilleos D S,Hatton T A,Vamvakaki M.Light-regulated supramolecular engineering of polymeric nanocapsules[J]. Journal of the American Chemical Society, 2011, 134(13): 5726-5729. |
| [57] | Vogel A, Venugopalan V. Mechanisms of pulsed laser ablation of biological tissues[J]. Chemical Reviews, 2003, 103(2): 577-644. |
| [58] | Susana C R, Markus O, Pilar R G, Carolin G, Pavlov A M, Sukhorukov G B, Parak W J. NIR-light triggered delivery of macromolecules into the cytosol[J]. Journal of Controlled Release, 2012, 159(1): 120-127. |
| [59] | Kurapatia R, Raichur A M. Near-infrared light-responsive graphene oxide composite multilayer capsules: a novel route for remote controlled drug delivery[J]. Chemical Communications, 2013, 49(7): 734-736. |




