光动力治疗(photodynamic therapy,PDT)作为一种非侵入性治疗手段,已被广泛用于许多癌症的临床治疗。其工作原理是利用光敏剂(photosensitizer,PS)在光照下产生的活性氧物种(reactive oxygen species,ROS)杀灭癌细胞[1, 2, 3, 4, 5, 6]。根据ROS种类与产生方式的不同,PDT可划分为Ⅰ型和Ⅱ型两种机制。I型机制中,激发态光敏剂与生物分子直接发生电子转移作用,产生自由基物种,这些自由基可进一步与氧气反应生成含氧自由基(如超氧阴离子自由基、羟基自由基等);Ⅱ型机制中,激发态光敏剂与氧气发生能量传递作用,产生单重态氧(1O2)[7, 8, 9, 10]。无论是含氧自由基还是单重态氧,都具有极高的反应活性,可以损伤多种生物分子,进而杀灭肿瘤细胞。
与肿瘤的三大传统治疗方式(手术、放射疗法和化学疗法)相比,PDT的主要优势在于其低的毒副作用[11]。光敏剂在暗环境下无法产生ROS,对细胞及组织并不表现出毒性[12]。通过对光照区域的精细控制,可以将PDT过程局限在肿瘤组织,实现对肿瘤细胞的高选择性杀伤[13, 14]。
然而,在临床应用中,PDT也面临多方面的挑战,光线在组织中的穿透深度就是其中之一。受光穿透深度的限制,PDT的临床应用仅限于体表及内窥镜可及区域(如皮肤癌、颈癌、口腔癌等)[15, 16, 17, 18, 19]。即便如此,对于浅表区域较大的实体肿瘤,PDT疗效也受到一定程度的抑制,这是因为绝大多数PS在紫外和可见光的蓝、绿波段有强的吸光能力,但这些波段的光也能被血红蛋白、黑色素以及体内其它含色素的蛋白质有效吸收,致使这一波段的辐照无法到达肿瘤组织内部。相比之下,波长在650~1100 nm的光具有良好的组织穿透深度,被称为理想的“光疗窗口”或“近红外(nearinfrared,NIR)窗口”。为此,许多研究着眼于发展在NIR波段强吸光的新型光敏剂。例如,Shen Z等[20]合成了一种BODIPY光敏剂,其在680~750 nm有强吸收,可以在NIR照射下产生PDT疗效。遗憾的是NIR光敏剂的合成往往非常复杂;相比传统光敏剂,1O2产率也较低。近年来,上转换纳米颗粒的快速发展,为解决PDT面临的光线穿透深度问题提供了新的途径。
2 镧系掺杂的上转换纳米颗粒上转换纳米颗粒(upconversion nanoparticles,UCNPs),由于可以将两个甚至更多的NIR光子转换为一个高能的光子,在过去十多年获得广泛关注[21, 22, 23]。UCNPs通常是由过渡金属(3d、4d、5d)、镧系金属(4f)或锕系金属(5f)离子掺杂于无机晶体基质中构成的。在这3类掺杂离子中,三价的镧系金属离子(La3+、Ce3+、Yb3+和Lu3+除外)由于具有多个亚稳能态,有利于上转换过程的发生,因而是最常用的掺杂离子。除了其独特的上转换发光性质外,镧系掺杂的UCNPs还凭借其它众多优势,包括弱的光漂白、可忽略的自发光背景、NIR强的组织穿透能力,以及非常小的光损伤,在医学成像领域展现出诱人的应用前景,成为一些传统光学成像材料(如有机染料、量子点等)的升级换代产品[22]。
上转换过程大致可分为3类:激发态吸收(excited state absorption,ESA)、能量传递上转换(energy transfer upconversion,ETU)和光子雪崩(photon avalanche,PA)。与双光子或多光子过程中多个光子的吸收同时发生不同,上述3种过程中光子的吸收是相继发生的,因而具有更高的上转换效率,无需借助高能脉冲激光,中等甚至较弱强度的连续激光就可实现这些上转换过程。为了提高上转换效率,通常还会掺杂一种在近红外区域具有较大吸收截面的离子作为敏化剂。Yb3+由于只具有一个激发4f态,从2F7/2到2F5/2的能量跃迁使其在980 nm处具有很大的吸收截面,因此是十分常用的吸光离子。图1给出了以Yb3+为光敏剂,以Er3+、Tm3+或Ho3+为上转换离子的UCNPs的上转换能量传递机制。
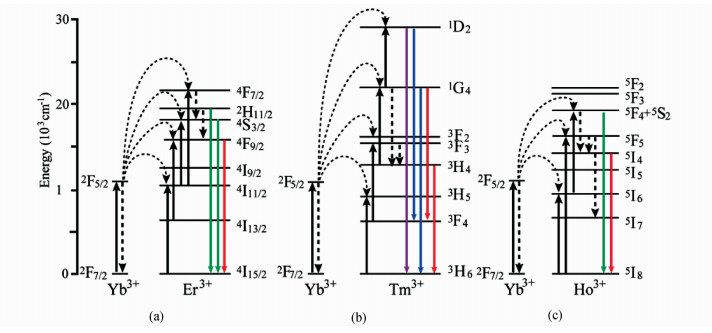 | 图1 基于Yb3+和Er3+、Tm3+或Ho3+的上转换纳米颗粒的上转换能量传递机制 Upconversion energy transfer mechanism based on Yb3+ and Er3+,Tm3+, Ho3+ doped UCNPs |
由于其结构和性质上的可“裁剪”特性,UCNPs可集多种影像功能于一身。例如,Yb3+和Gd3+离子掺杂可以赋予UCNPs体系X射线体层摄影(X-ray computed tomography,CT)造影剂能力;Gd3+掺杂使UCNPs体系在光学影像基础上兼备磁共振成像功能[22, 24]。
除了成像诊断方面的应用外,UCNPs在治疗方面也得到了诸多应用。同其它纳米材料一样,UCNPs有着大的比表面积及其丰富的可修饰特性,可作为药物输送载体加以应用[25, 26]。更具特色的则是利用UCNPs的上转换发光特性,将其作为一种光能转换器件,应用于PDT或其它光响应药物释放体系[27, 28, 29]。
3 以UCNPs为基础的光动力治疗研究借助UCNPs上转换发光特性,可以使传统的可见光甚至紫外光激发的光敏剂在NIR激发下呈现出PDT疗效[30],有助于实现深层肿瘤的治疗。以UCNPs为基础的光动力治疗研究发展极为迅速,表 1列举了近年来的一些代表性工作。
| 表1 980 nm激发的UCNPs-PDT体系 UCNPs-PDT systems excited at 980 nm |
从表1可以看出,所报道的UCNPs-PDT体系中所用的光敏剂绝大多数都是基于单重态氧机制,如卟啉类染料TPP、Ce6、焦脱镁叶绿酸a、酞菁类染料ZnPc、二羟基硅酞菁,以及其它结构多样的光敏染料,如菁染料MC540、亚甲基蓝MB、竹红菌甲素、玫瑰红(RB)和富勒烯等。表1中的UCNP-TiO2较为独特,该体系在近红外光激发下能够产生羟基自由基。
为了提高UCNPs-PDT对肿瘤细胞的选择性,许多体系的颗粒表面修饰上靶向功能分子,如叶酸、RGD肽、抗体等。此外,为使UCNPs-PDT在水溶液中具有良好的分散性,人们尝试了多种方法,包括各种方式的二氧化硅包覆以及聚合物(如PEG、壳聚糖、PEI)包覆等。
4 以上转换纳米颗粒和光动力治疗为基础的肿瘤联合疗法目前,肿瘤治疗的一个最大挑战来自于肿瘤细胞的耐药性,这使得许多经典抗肿瘤药物或疗法难于根除病灶[43, 44]。同时输送两种或更多种治疗药物,或者将不同治疗方法相结合而发展起来的联合疗法(combination therapy)[45, 46],由于面向非单一靶点,且借助各类药物和疗法间的协同作用,往往会取得单一药物和疗法难以企及的疗效。此外,联合疗法中各种药物的用量显著低于单一疗法,显著降低了药物的毒副作用[47]。过去3年,对于UCNPs在治疗方面的应用已经从单功能治疗转移到联合疗法。例如Zhao Y小组[48]首先利用UCNPs同时传递化疗药物和光敏剂实现化疗与光动力治疗的结合。Zhang Y等[49]则在UCNPs上同时负载两种光动力药物,发现抗肿瘤效果比单独负载一种更为有效。这些研究为UCNPs-PDT研究开启了一片新的空间。本文综述了近年来以UCNPs-PDT为基础的联合疗法的研究进展,并分析了这一新兴领域面临的挑战和机遇,以此来吸引更多研究者参与该领域的发展。
构建UCNPs治疗体系的主要考虑因素是将UCNPs与其它功能组分(包括无机材料和有机分子)结合到一个纳米体系中。首先,为了满足生物及医药应用的要求,有必要对UCNPs表面进行水溶性改性[24],这不仅可以解决UCNP生物相容性问题,同时也为负载治疗药物提供有效位点[25, 26]。Yang等总结了UCNPs表面水溶性改性的常见策略:(1)包覆致密的或介孔的二氧化硅薄层(UCNPs@SiO2);(2)通过共价键合或者非共价自组装方式包覆生物相容性高分子材料(UCNPs@polymer)。此外,靶向功能基团,如叶酸、缩氨酸、抗体等也常被负载到UCNPs表面,用以提高药物体系对肿瘤细胞的选择性[22]。
UCNPs具有天然的光学成像潜力。除此之外,利用镧系元素离子半径与化学性质相似的特性,可以通过掺杂多种镧系离子的方法实现多模式成像,这无疑为示踪药物的输运、吸收、释放以及疗效提供了可能。例如,镱、镥掺杂的UCNPs不仅可以用作计算机断层扫描造影剂,还能作为放疗增敏剂用于肿瘤的放射治疗[50];钆为基底或者掺杂的UCNPs兼备核磁成像(MRI)、上转换荧光成像及中子俘获治疗潜力。UCNPs本身就兼备诊疗双重功能的特点,使之成为一类非常理想的联合疗法平台。
下面简述近年来以UCNPs-PDT为基础的联合疗法研究的最新进展。
4.1 化疗与光动力疗法的结合化疗与PDT的联合疗法在临床前研究与临床研究方面均具有很长的历史[51]。化疗与PDT的协同作用表现在:PDT产生的ROS可以抑制外排蛋白的表达,从而抑制化疗药物外排;与此同时,化疗药物可以提高肿瘤细胞对PDT的敏感性[52]。Zhao Y等[48]首次利用α-环糊精修饰的UCNPs同时负载光敏剂和化疗药物阿霉素(doxorubicin,DOX)(图2),利用980nm光照实现PDT,并利用肿瘤的弱酸性微环境实现阿霉素释放。该工作中使用的Mn2+掺杂的NaYF4∶Yb/Er UCNPs产生单波段红光发射[53],而负载的光敏剂二氢卟酚 e6(chlorin e6,Ce6)、酞菁锌(zinc phthalocyanine,ZnPc)或亚甲基蓝(methylene blue,MB)[54]均可以有效吸收630~700 nm发光。与仅负载光敏剂体系比较,同时负载Ce6和DOX体系在980 nm激光照射下对A549细胞表现出更强的细胞毒性。
上述工作中,只有单重态氧的产生是光响应过程,化疗药物的释放与光照与否无关。最近,Yuan Y等利用可被紫外光断裂的基团将DOX连接到聚乙二醇化的共轭聚电解质(conjugated polyelectrolyte,CPE)上,以此来封装NaYF4∶Yb/Tm UCNPs[55]。980 nm激光照射,上转换紫外光可以光解邻硝基苄基基团,实现化疗药物释放[56];同时,上转换可见光可激发共轭聚电解质产生单重态氧,实现PDT (图3a)。Yang S等[57]则设计了一种单重态氧诱导的化疗药物释放体系。如图3b所示,Mn2+掺杂的NaYF4:Yb/Er UCNPs首先包裹一层介孔二氧化硅,随后表面修饰长烷基链和光敏剂Ce6,再通过自组装过程包覆一层单重态氧响应的两亲性共聚物,并将DOX包覆其中。980 nm光照射后,上转换红光可以有效激发Ce6产生单重态氧,用于光动力治疗;此外,两亲性共聚物中的9,10-二烷氧基蒽基团被单重态氧氧化为9,10蒽醌[58],导致聚合物降解并释放DOX。荷瘤小鼠注射复合体系药物并经激光照射后,肿瘤生长速度明显变慢,表现出显著的光动力疗法与化疗的协同作用。
 | 图3 Yuan Y等[55](a)和Yang S等[57](b)报道的UCNPs-PDT联合治疗体系 UCNPs-PDT combination therapy systems reported by Yuan Y et al[55](a) and by Yang S et al[57] (b) |
表 1中所示的UCNPs-PDT体系局限于使用一种光敏剂[27, 59]。然而,UCNPs可同时有多种颜色的上转换发光,这意味着只有一部分上转换发光被利用60。为了充分利用上转换发光来增强光动力治疗效果,Zhang Y等[49]将两种光敏剂同时负载到UCNPs上(图4a)。在980 nm光照射下,NaYF4∶Yb/Er UCNPs的上转换绿光与部花青素540(MC-540)光谱重叠,而上转换红光与ZnPc吸收光谱重叠(图4b),使得两种光敏剂被同时激发成为可能。体外PDT实验表明:相比单一光敏剂体系,双光敏剂体系抗肿瘤效率更高。随后,作者对复合体系修饰叶酸(FA),并对患有B16-F0黑素瘤的C57BL/6小鼠进行NIR光动力治疗,肿瘤生长得到明显抑制。
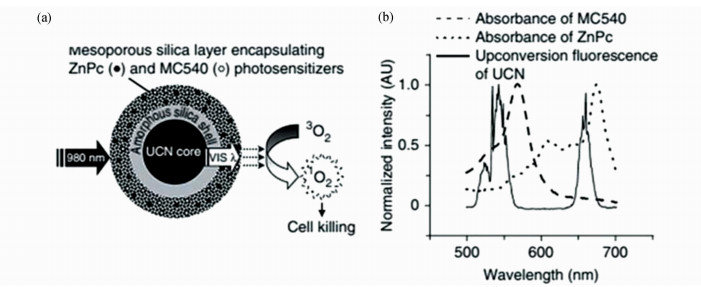 | 图4 (a) Zhang Y等报道的UCNPs-PDT联合治疗体系;(b) UCNPs上转换发光光谱以及ZnPc和MC540的吸收光谱[49] (a) UCNPs-PDT combination therapy system reported by Zhang Y et al; (b) Upconversion emission spectrum of the UCNPs and absorption spectra of ZnPc and MC540[49] |
光热疗法(photothermal therapy,PTT)与PDT类似,也是一种非侵入性光疗手段[61]。不同的是,PDT依赖ROS杀死肿瘤细胞,PTT则是将光能转换为热能,“烧死”肿瘤细胞[62]。由于适当热效应可加快瘤内血液流动,有利于氧气向肿瘤内部的输送,从而促进PDT效率,所以PTT与PDT的联合会表现出协同作用[63]。Wang Y等[64]设计了基于UCNPs的PTT-PDT联合治疗体系,他们利用具有光热效应的纳米氧化石墨烯(nano-graphene oxide,NGO)负载壳核结构的NaYF4∶Yb/Er/Tm@NaYF4 UCNPs和光敏剂ZnPc,980 nm光照射下体系呈现高对比度上转换荧光,可用于药物体系的实时监测;630 nm光照下,光敏剂产生单重态氧,表现出PDT活性;808 nm光照射下,体系则表现出PTT活性。与单独PTT或PDT相比,联合体系的治疗效果显著提高。在该体系中,UCNPs仅起到光学成像作用,光敏剂的激发并非来自其上转换发光。
随后,Chen Q等[65]将玫瑰红(Rose Bengal,RB)和一种NIR染料(IR825)同时负载到牛血清白蛋白修饰的NaGdF4∶Yb/Er UCNPs上(图5a),RB吸收UCNPs的上转换绿光,通过PDT作用杀死肿瘤细胞,而IR825在808 nm光照射下实现PTT。由于PTT和PDT的协同作用,肿瘤细胞
几乎被全部杀死,与单一980 nm光照和808 nm光照对比,肿瘤杀伤效果显著提高(图5b)。PTT+PDT的体内抑瘤效果依然明显。
 | 图5 (a) Chen Q等报导的UCNPs-PDT联合治疗体系;(b) PDT、PTT及PTT + PDT体外细胞毒性[65] (a) UCNPs-PDT combination therapy system reported by Chen Q et al;(b)Cytotoxicity of PTD, PTT, and PDT+PTT in vitro[65] |
上述研究中,两种激光分别被用于PDT和PTT。为了操作上的简易性以及患者的舒适性,利用一种激光实行PDT和PTT则更具优势[61]。Yin M等[66]将具有光热效应的CuS纳米颗粒[67]修饰到MB掺杂的二氧化硅包覆的NaYF4∶Mn/Yb/Er UCNPs,980 nm光照下,MB会被上转换红光激发,产生1O2,而CuS纳米颗粒也可以吸收980nm激光并将其转化为热量,结果复合体系对革兰氏阴性及阳性菌产生明显的抗菌效果。
4.4 基因疗法与光动力疗法的结合基因疗法是利用遗传物质,如DNA或小分子干扰RNA(siRNA),来治疗或防御疾病的方法,已被持续广泛而深入地研究了数十年之久[68]。具有治疗效果的siRNA可通过纳米载体被有效输送到细胞当中降解目标mRNA,从而使目标蛋白沉默[69]。目前,以纳米颗粒为基础的基因输送主要受溶酶体逃逸的限制[70]。光化学内化转染技术已被证明是改善细胞内基因输送的有效方式。它包括利用特定波长的光照射,使嵌入到细胞核内体(endosome)或溶酶体的光敏剂产生ROS,从而扰乱核内体或溶酶体膜,实现溶酶体逃逸和进一步的基因表达[71]。Jayakumar M K等[72]利用介孔二氧化硅包覆的NaYF4∶Yb/Tm@NaYF4∶Yb/Er来实现PDT与基因疗法的协同。如图6所示,上转换发光的紫外部分可以激发光吗啉基(吗啉基为短的核酸类似物,其非常稳定且可有效实现基因沉默,而光吗啉基是光敏吗啉基,在UV照射后表现出活性),可见光部分可以激发光敏剂间四苯基喹啉(meso-tetraphenylporphyrin,TPPS2a)来扰乱溶酶体膜,从而增强药物摄取。患有C57BL6/J的B16F0小鼠,在注射负载有抑制SATA3的光吗啉基和TPPS2a的UCNPs后,经NIR照射,可以看到显著增强的细胞药物摄取和STAT3沉默。而SATA3的沉默导致小鼠肿瘤体积显著减少。
 | 图6 Jayakumar M K等[72]报道的UCNPs-PDT联合治疗体系 UCNPs-PDT combination therapy system reported by Jayakumar M K et al[72] |
随后,Wang Y等[73]利用带大量正电荷的聚乙烯胺(polyethylenimine,PEI)修饰的NaGdF4∶Yb/Er UCNPs负载光敏剂Ce6和siRNA来实现PDT与基因治疗的协同。其输送的siRNA可以靶向激酶1(Plk1)致癌基因(此基因在多种癌细胞中过表达并且在DNA复制过程中扮演关键角色),而静默Plk1可以诱导细胞凋亡[74]。在其报道当中,注射UCNP-PEG@2×PEI-Ce6-siPlk1复合体系并随后经980 nm光照射的HeLa细胞表现出PDT与基因治疗的协同作用。
4.5 放疗与光动力疗法的结合放射疗法(radiotherapy,RT)是利用高能X射线或者γ射线照射肿瘤部位非侵入性地产生具有细胞毒性的光生电子和自由基来杀死肿瘤细胞[75]。然而,肿瘤细胞含氧量低的特点使得放疗效率低下,因此不得不通过提升放射剂量来提高治疗效率,这可能会超过周围正常细胞所能承受的范围。辐射敏化剂可一定程度增强放疗效果,被广泛用来改善乏氧肿瘤细胞对辐射的敏感性[76]。近年来,许多高阻抗材料如金属纳米颗粒、量子点、一些化疗药物被用作辐射光敏剂来增强乏氧肿瘤细胞的放疗效果[50]。
Shi J L等[77]率先利用UCNPs作为辐射敏化剂提升放疗效果,并且将此疗法与PDT相结合。他们以NaYF4∶Yb/Er/Tm@NaGdF4 UCNPs为核,以多孔二氧化硅为壳层,二者之间通过表面保护刻蚀形成可控的内腔,如图7a。一种辐射敏化剂分子血卟啉(hematoporphyrin,HP)被共价嫁接到二氧化硅层内形成UCMSNs-HP复合体系,在X射线和980 nm激光照射下实现PDT/RT联合治疗(如图7b),既克服了单独PDT治疗深度有限的缺点,又对RT起到了增敏作用[78]。研究还表明,经X射线照射的肿瘤细胞对单重态氧更加敏感[59]。
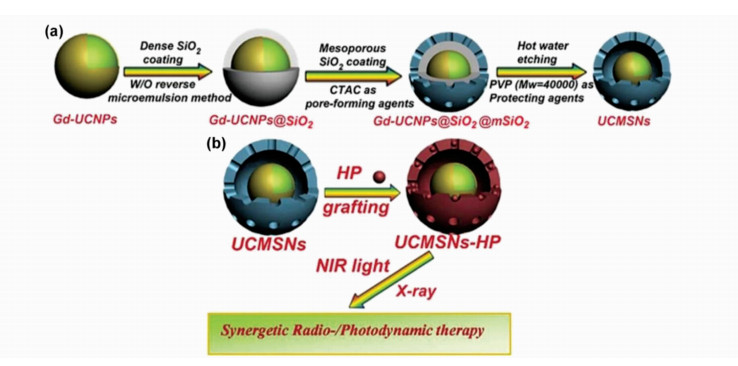 | 图7 (a)UCMSNs合成步骤;(b) UCMSNs-HP联合治疗图示[77] (a)Synthetic routes of UCMSNs; (b) Schematic illustration of UCMSNs-HP combination therapy[77] |
近年来,以UCNPs为基础的三模式治疗体系也已被报道。Shi J L等[77]利用上述的UCMSNs同时负载辐射敏化的光敏剂HP,辐射敏化的化疗药物多烯紫杉醇(docetaxel,Dtxl),在980 nm激光及X射线照射下实现化疗/放疗/光动力疗法三模式协同(图8a)。该体系最大的特点是在极大降低药物剂量的同时,保证了有效的抗癌效果[46]。
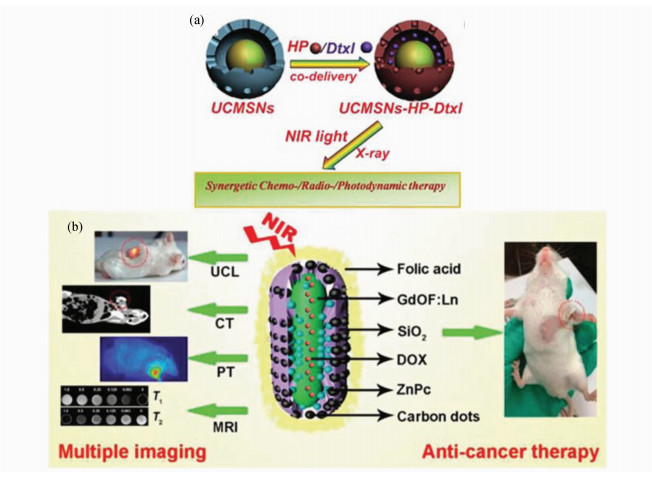 | 图8 (a)UCMSNs-HP-Dtxl联合治疗体系[77];(b)GdOF∶Ln@SiO2-ZnPc-CDs-DOX联合治疗体系[79] (a) UCMSNs-HP-Dtxlcombination therapy system[77]; (b) GdOF∶Ln@SiO2-ZnPc-CDs-DOX combination therapy system[79] |
最近Lü R等[79]设计了一种多功能的GdOF:Yb/Er/Mn@SiO2的蛋黄结构的微囊,由光敏剂ZnPc及光热碳量子点修饰,并同DOX一同封装,实现PDT/PTT/化疗的联合疗法(图8b)。在980 nm激光照射下,上转换红光可以激发ZnPc产生单重态氧,用于PDT;而壳外碳量子点可将NIR转换为热能,实现PTT[80];同时,局部的热效应有利于DOX的释放。
5 问题与展望虽然基于UCNPs-PDT的联合疗法研究近年来得到长足的发展,但面向临床应用,该领域还需正视多方面的挑战,这无疑成为其未来研究和发展的方向。
5.1 合理选择药物、疗法和治疗方案联合疗法的目的是借助不同药物或疗法间的协同作用,构建更为高效的治疗体系,在提高疗效和抑制耐药性的同时,降低单一药物和治疗手段的毒副作用。药物或疗法的联合并不是随意的,能否获得协同效应是关键指标。此外,联合疗法还要考虑各种药物或疗法的时空特性。例如,在PDT/化疗联合治疗中,PDT在激光照射后通过快速产生1O2来杀死肿瘤细胞,但化疗药物的药效却缓慢得多,合理设计PDT时间点就显得十分重要。
5.2 有效抑制激光引发的光损伤基于UCNPs的光疗体系,光疗效果与激光强度和激光波长紧密相关。目前,UCNPs的上转换发光效率还远不理想,通常低于1%。因此,往往需要高强度NIR来激活光响应治疗体系,这势必会超过人类皮肤和组织对光照的可承受能力,从而引起严重的光损伤。目前,人们正在努力探寻提高UCNPs上转换量子产率的有效策略[81],包括操纵主晶格、调制能量传送、表面钝化、表面等离子体耦合、带宽敏化,以及光子晶体设计,这方面研究的飞跃有助于推动UCNPs的临床应用。
另一大问题来自于激光的波长。目前的UCNPs主要响应980 nm激光。但由于水在该波长有吸收,致使980 nm激光的热效应显著[81],可能对正常组织造成严重伤害。改变UCNPs的激光响应波长已成为该领域的研究热点。Zhan等[82]使用915 nm激光代替980 nm激光,显著降低了热效应。Zou W等[83]则利用NIR有机染料作为敏化剂,构建了一个宽激发带(740~850 nm)UCNPs。研究表明,利用Nd3+作为光敏剂离子,可以构建对808 nm响应的UCNPs[84],避免了生物组织中水对光的吸收,使其在生物应用方面更加安全。
5.3 进一步调控UCNPs的尺寸、形貌及表面态纳米颗粒的细胞摄取与一系列因素有关,包括颗粒大小、形貌、表面电荷以及表面化学性质[85]。虽然相关研究已有大量的报道[86, 87, 88],但涉及到UCNPs的工作还处于起步阶段。为了保证以UCNPs为基础的纳米治疗体系能够被肿瘤细胞高效捕获与摄取,急需深入研究UCNPs尺寸和形貌对疗效的影响,并探索新的表面修饰手段。
5.4 系统研究UCNPs的生物安全性UCNPs临床应用前还必须明确其系统毒性及体内的长期效应。虽然目前的研究表明UCNPs短期体内和体外实验均表现安全,但其长期毒性及富集特性尚未被探究[89, 90]。为实现UCNPs的临床应用,这些研究显然是必需的。
5.5 拓展UCNPs在光疗领域的应用范围除上面提到的光动力疗法PDT和光热疗法PTT外,近年来基于光活化的化学疗法(photoactivated chemotherapy,PACT)成为光疗领域一个新的备受关注的研究方向。目前作为PACT潜在试剂加以研究的多为过渡金属配合物,它们在光照后发生配体解离,生成的配位不饱和物种能够与生物活性分子(如DNA)共价结合,进而导致细胞死亡。该类配合物的吸收光谱多在500 nm以下。UCNPs的上转换特性无疑为该类药物体系的光活化波长向光疗窗口发展提供了机遇。
近期,我们利用超分子自组装将可见光活化的cis-[Ru(bpy)2(C18H37CN)2]2+负载到PEG包覆的UCNPs表面,利用NIR实现了cis-[Ru(bpy)2(H2O)2]2+的释放及其对DNA的共价结合[91]。这种简单的负载策略为构筑其它NIR活化的抗肿瘤金属药物提供了一种行之有效的策略。
致谢:感谢国家自然科学基金委员会的基金(21301182)支持| [1] | Shanmugam V, Selvakumar S, Yeh C S. Near-infrared light-responsive nanomaterials in cancer therapeutics[J]. Chemical Society Reviews, 2014, 43:6254-6287. |
| [2] | Cheng L, Wang C, Feng L, Yang K, Liu Z. Functional nanomaterials for phototherapies of cancer[J]. Chemical Reviews, 2014, 114:10869-10939. |
| [3] | Shi E S, Hamada M, Murase N, Bi V. Nanomaterials formulations for photothermal and photodynamic therapy of cancer[J]. Journal of Photochemistry and Photobiology C: Photochemistry Reviews, 2013, 15:53-72. |
| [4] | Master A, Livingston M, Gupta A S. Photodynamic nanomedicine in the treatment of solid tumors: perspectives and challenges[J]. Journal of Controlled Release, 2013, 168:88-102. |
| [5] | Brown S B, Brown E A, Walker I.The present and future role of photodynamic therapy in cancer treatment[J]. The Lancet Oncology, 2004, 5:497-508. |
| [6] | Zhu Z, Tang Z, Phillips J A.Yang R, Wang H, Tan W. Regulation of singlet oxygen generation using single-walled carbon nanotubes[J]. Journal of the American Chemical Society, 2008, 130:10856-10857. |
| [7] | Lucky S S, Soo K C, Zhang Y. Nanoparticles in photodynamic therapy[J]. Chemical Reviews, 2015, 115:1990-2042. |
| [8] | Lakshmanan S, Gupta G K, Avci P, Chandran R, Sadasivam M, Jorge A E S, Hamblin M R. Physical energy for drug delivery; poration, concentration and activation[J]. Advanced Drug Delivery Reviews, 2014, 71:98-114. |
| [9] | Nishiyama N, Morimoto Y, Jang W D, Kataoka K. Design and development of dendrimer photosensitizer-incorporated polymeric micelles for enhanced photodynamic therapy[J]. Advanced Drug Delivery Reviews, 2009, 61:327-338. |
| [10] | 雷万华,王雪松.光动力抗菌光敏剂的研究进展[J].影像科学与光化学, 2013, 31(5):321-334. Lei W H, Wang X S. Research progress of photodynamic antimicrobial photosensitizers[J]. Imaging Science and Photochemistry, 2013, 31(5):321-334. |
| [11] | Lin L, Xiong L, Wen Y, Lei S, Deng X, Liu Z, Chen W, Miao X. Active targeting of nano-photosensitizer delivery systems for photodynamic therapy of cancer stem cells[J]. Journal of Biomedical Nanotechnology, 2015, 11:531-554. |
| [12] | Kim J, Tung C H, Choi Y.Smart dual-functional warhead for folate receptor-specific activatable imaging and photodynamic therapy[J]. Chemical Communications, 2014,50:10600-10603. |
| [13] | Yuan Y, Feng G, Qin W, Tang B Z, Liu B. Targeted and image-guided photodynamic cancer therapy based on organic nanoparticles with aggregation-induced emission characteristics[J]. Chemical Communications, 2014, 50:8757-8760. |
| [14] | Chen M H, Ji L N, Mao Z W. Cyclometalated Ir(Ⅲ) complexes as targeted theranostic anticancer therapeutics: combining HDAC inhibition with photodynamic therapy[J]. Chemical Communications, 2014, 50:10945-10948. |
| [15] | Lu K, He C, Lin W. Nanoscale metal-organic framework for highly effective photodynamic therapy of resistant head and neck cancer[J]. Journal of the American Chemical Society, 2014, 136:16712-16715. |
| [16] | Synatschke C V, Nomoto T, Cabral H, Foörtsch M, Toh K, Matsumoto Y, Miyazaki K, Hanisch A, Schacher F H, Kishimura A.Multicompartment micelles with adjustable poly (ethylene glycol) shell for efficient in vivo photodynamic therapy[J]. ACS Nano, 2014, 8:1161-1172. |
| [17] | Sosnik A, Carcaboso A M.Nanomedicines in the future of pediatric therapy[J]. Advanced Drug Delivery Reviews, 2014, 73:140-161. |
| [18] | Ho M, Lo E, Young A, Liu D.Outcome of polypoidal choroidal vasculopathy at 1 year by combined therapy of photodynamic therapy with ranibizumab and predictive factors governing the outcome[J]. Eye, 2014, 28:1469-1476. |
| [19] | Erikitola O, NwaobiR, Lotery A, Sivaprasad S.Photodynamic therapy for central serous chorioretinopathy[J]. Eye, 2014,28:944-957. |
| [20] | Yang Y, Guo Q, Chen H, Zhou Z, Guo Z, Shen Z. Thienopyrrole-expanded BODIPY as a potential NIR photosensitizer for photodynamic therapy[J]. Chemical Communications, 2013, 49:3940-3942. |
| [21] | Auzel F. Upconversion and anti-Stokes processes with f and d ions in solids[J]. Chemical Reviews, 2004, 35:139-173. |
| [22] | Zhou J, Liu Q, Feng W, Sun Y, Li F Y. Upconversion nanophosphors for small-animal imaging[J]. Chemical Society Reviews, 2012, 41:1323-1349. |
| [23] | Liu X G, Yan C H, John C. A. Photon upconversion nanomaterials[J]. Chemical Society Reviews, 2015, 44:1299-1301. |
| [24] | Liu Q, Feng W, Li F. Water-soluble lanthanide upconversion nanophosphors: Synthesis and bioimaging applications in vivo[J]. Coordination Chemistry Reviews, 2014, 273:100-110. |
| [25] | Yang D M,Ma P A,Hou Z Y,Chen Z Y,Li C X,Lin J.Current advances in lanthanide ion (Ln3+)-based upconversion nanomaterials for drug delivery[J]. Chemical Society Reviews, 2015, 44(6):1416-1448. |
| [26] | Wu X, Chen G,Shen J, Li Z, Zhang Y,Han G. Upconversion nanoparticles: a versatile solution to multiscale biological imaging[J]. Bioconjugate Chemistry, 2015,26:166-175. |
| [27] | Chao W, Liang C, Zhuang L.Upconversion nanoparticles for photodynamic therapy and other cancer therapeutics[J]. Theranostics, 2013, 3:317-330. |
| [28] | Niagara Muhammad I, Akshaya B, Yong Z.Upconversion nanoparticles as versatile light nanotransducers for photoactivation applications[J]. Chemical Society Reviews, 2014, 44:1449-1478. |
| [29] | Min Y Z,Li J M, Liu F, Yeow E, Xin B G. Near-infrared light-mediated photoactivation of a platinum antitumor prodrug and simultaneous cellular apoptosis imaging by upconversion-luminescent nanoparticles[J]. Angewandte Chemie International Edition, 2014, 53:1030-1034. |
| [30] | Dou Q Q, Rengaramchandran A, Selvan S T, Paulmurugan R, Zhang Y. Core-shell upconversion nanoparticle-semiconductor heterostructures for photodynamic therapy[J]. Scientific Reports, 2015, 5:1-8. |
| [31] | Zhang P, Steelant W, Kumar M, Scholfield M. Versatile photosensitizers for photodynamic therapy at infrared excitation[J]. Journal of the American Chemical Society, 2007, 129:4526-4527. |
| [32] | Ungun B, Prud'Homme R K, Budijon S J, Shan J, Lim S F, Ju Y, Austin R. Nanofabricated upconversion nanoparticles for photodynamic therapy[J]. Optics Express, 2009, 17:80-86. |
| [33] | Qian H S, Guo H C, Ho P C L, Mahendran R, Zhang Y. Mesoporous-silica-coated up-conversion fluorescent nanoparticles for photodynamic therapy[J]. Small, 2009, 5:2285-2290. |
| [34] | Wang C, Tao H, Cheng L, Liu Z. Near-infrared light induced in vivo photodynamic therapy of cancer based on upconversion nanoparticles[J]. Biomaterials, 2011, 32:6145-6154. |
| [35] | Liu K, Liu X, Zeng Q, Zhang Y, Tu L, Liu T, Kong X, Wang Y, Cao F, Lambrechts S A. Covalently assembled NIR nanoplatform for simultaneous fluorescence imaging and photodynamic therapy of cancer cells[J]. ACS Nano, 2012, 6:4054-4062. |
| [36] | Tu D, Liu Y, Zhu H, Chen X. Optical/magnetic multimodal bioprobes based on lanthanide-doped inorganic nanocrystals[J]. Chemistry-A European Journal, 2013, 19:5516-5527. |
| [37] | Xu Q C, Zhang Y, Tan M J, Liu Y, Yuan S, Choong C, Tan N S, Tan T T Y. Anti-cAngptl4 Ab-conjugated N-TiO2/NaYF4:Yb, Tm nanocomposite for near infrared-triggered drug release and enhanced targeted cancer cell ablation[J]. Advanced Healthcare Materials, 2012,1(4):470-474. |
| [38] | Zhou A, Wei Y, Wu B, Chen Q, Xing D. Pyropheophorbide A and c (RGDyK) comodified chitosan-wrapped upconversion nanoparticle for targeted near-infrared photodynamic therapy[J]. Molecular Pharmaceutics, 2012, 9:1580-1589. |
| [39] | Qiao X F, Zhou J C, Xiao J W, Wang Y F, Sun L D, Yan C H. Triple-functional core-shell structured upconversion luminescent nanoparticles covalently grafted with photosensitizer for luminescent, magnetic resonance imaging and photodynamic therapy in vitro[J]. Nanoscale, 2012, 4:4611-4623. |
| [40] | Liu X, Zheng M, Kong X, Zhang Y, Zeng Q, Sun Z, Buma W J, Zhang H. Separately doped upconversion-C 60 nanoplatform for NIR imaging-guided photodynamic therapy of cancer cells[J]. Chemical Communications, 2013, 49:3224-3226. |
| [41] | Jin S, Zhou L, Gu Z, Tian G, Yan L, Ren W, Yin W, Liu X, Zhang X, Hu Z. A new near infrared photosensitizing nanoplatform containing blue-emitting up-conversion nanoparticles and hypocrellin A for photodynamic therapy of cancer cells[J]. Nanoscale, 2013, 5:11910-11918. |
| [42] | Yuan Q, Wu Y, Wang J, Lu D, Zhao Z, Liu T, Zhang X, Tan W. Targeted bioimaging and photodynamic therapy nanoplatform using an aptamer-guided G-Quadruplex DNA carrier and near-infrared light[J]. Angewandte Chemie International Edition, 2013, 52:13965-13969. |
| [43] | Chou T C. Drug combination studies and their synergy quantification using the chou-talalay method[J]. Cancer Research, 2010, 70:440-446. |
| [44] | Xin D, Tan C. Combination of microRNA therapeutics with small-molecule anticancer drugs: mechanism of action and co-delivery nanocarriers[J]. Advanced Drug Delivery Reviews, 2015, 81:184-197. |
| [45] | Lehár J, Krueger A S, Avery W, Heilbut A M, Johansen L M, Price E R, Rickles R J, ShortIIii G F, Staunton J E, Jin X. Synergistic drug combinations tend to improve therapeutically relevant selectivity[J]. Nature Biotechnology, 2009, 27:659-666. |
| [46] | Greco F, Vicent M J. Combination therapy: opportunities and challenges for polymer-drug conjugates as anticancer nanomedicines[J]. Advanced Drug Delivery Reviews, 2009, 61:1203-1213. |
| [47] | Lammers T. Improving the efficacy of combined modality anticancer therapy using HPMA copolymer-based nanomedicine formulations[J]. Advanced Drug Delivery Reviews, 2010, 62:203-230. |
| [48] | Tian G, Ren W, Yan L, Jian S, Gu Z, Zhou L, Jin S, Yin W, Li S, Zhao Y. Red-emitting upconverting nanoparticles for photodynamic therapy in cancer cells under near-infrared excitation[J]. Small, 2013,9:1929-1938. |
| [49] | Idris N M, Gnanasammandhan M K, Zhang J, Ho P C, Mahendran R, Zhang Y. In vivo photodynamic therapy using upconversion nanoparticles as remote-controlled nanotransducers[J]. Nature Medicine, 2012, 18:1580-1585. |
| [50] | Xing H, Zheng X, Ren Q, Bu W, Ge W, Xiao Q, Zhang S, Wei C, Qu H, Wang Z. Computed tomography imaging-guided radiotherapy by targeting upconversion nanocubes with significant imaging and radiosensitization enhancements[J]. Scientific Reports, 2013, 3:1-9. |
| [51] | Severyn B, Liehr R A, Wolicki A, Nguyen K H, Hudak E M, Ferrer M, Caldwell J S, Hermes J D, Jing L, Tudor M. Parsimonious discovery of synergistic drug combinations[J]. ACS Chemical Biology, 2011, 6:1391-1398. |
| [52] | Chen Q, Wang X, Wang C, Feng L, Li Y, Liu Z. Drug-induced self-assembly of modified albumins as nano-theranostics for tumor-targeted combination therapy[J]. ACS Nano, 2015,9:5223. |
| [53] | Tian G, Gu Z J, Zhou L J,Yin W Y,Liu X X,Yan L,Jin S,Ren W L,Xin G M,Li S J.Mn2+ dopant-controlled synthesis of NaYF4:Yb/Er upconversion nanoparticles for in vivo imaging and drug delivery[J]. Advanced Materials, 2012, 24(9):1226-1231. |
| [54] | Chatterjee D, Fong L, Zhang Y. Nanoparticles in photodynamic therapy: an emerging paradigm[J]. Advanced Drug Delivery Reviews, 2008, 60:1627-1637. |
| [55] | Yuan Y, Min Y, Hu Q, Xing B, Liu B. NIR photoregulated chemo-and photodynamic cancer therapy based on conjugated polyelectrolyte-drug conjugate encapsulated upconversion nanoparticles[J]. Nanoscale, 2014, 6(19):11259-11272. |
| [56] | Yuan H X,Chong H,Wang B,Zhu C L,Liu L B,Yang Q,Lv F T,Wang S. Chemical molecule-induced light-activated system for anticancer and antifungal activities[J]. Journal of the American Chemical Society, 2012, 134(32):13184-13187. |
| [57] | Yang S,Li N J,Liu Z,Sha W W,Chen D J,Xu Q F,Lu J M. Amphiphilic copolymer coated upconversion nanoparticles for near-infrared light-triggered dual anticancer treatment[J]. Nanoscale,2014, 6(24):14903-14910. |
| [58] | Dumitru A, Larisa K, Andriy M. 1,9-Dialkoxyanthracene as a 1O2-sensitive linker[J]. Journal of the American Chemical Society, 2011, 133(11), 3972-3980. |
| [59] | Lucky S S, Soo K C, Zhang Y. Nanoparticles in photodynamic therapy[J]. Chemical Reviews, 2015, 115:1990-2042. |
| [60] | Liu X M,Zheng M,Kong X G,Zhang Y L,Zeng Q H,Sun Z C,Buma W J,Zhang H. Separately doped upconversion-C60 nanoplatform for NIR imaging-guided photodynamic therapy of cancer cells[J]. Chemical Communications, 2013, 49(31):3224-3226. |
| [61] | Cheng L, Wang C, Feng L Z, Yang K, Liu Z. Functional nanomaterials for phototherapies of cancer[J]. Chemical Reviews, 2014, 114(21):10869-10939. |
| [62] | Yong Y,Zhou L J,Gu Z J,Yan L,Tian G,Zheng X P,Liu X D,Zhang X,Shi J X,Cong W S. WS2 nanosheet as a new photosensitizer carrier for combined photodynamic and photothermal therapy of cancer cells[J]. Nanoscale, 2014, 6(17):10394-10403. |
| [63] | Bo T, Chao W, Shuai Z, Feng L, Zhuang L. Photothermally enhanced photodynamic therapy delivered by nano-graphene oxide[J]. ACS Nano, 2011, 5:7000-7009. |
| [64] | Wang Y, Wang H, Liu D, Song S, Xiao W, Zhang H. Graphene oxide covalently grafted upconversion nanoparticles for combined NIR mediated imaging and photothermal/photodynamic cancer therapy[J]. Biomaterials, 2013, 34:7715-7724. |
| [65] | Chen Q, Wang C, Cheng L, He W, Cheng Z, Liu Z. Protein modified upconversion nanoparticles for imaging-guided combined photothermal and photodynamic therapy[J]. Biomaterials, 2014, 35:2915-2923. |
| [66] | Yin M, Li Z, Ju E, Wang Z, Dong K, Ren J, Qu X. Multifunctional upconverting nanoparticles for near-infrared triggered and synergistic antibacterial resistance therapy[J]. Chemical Communications, 2014, 50:10488-10490. |
| [67] | Xiao Z Y. Cu S nanoparticles: clinically favorable materials for photothermal applications[J]. Nanomedicine, 2014, 9(3): 373-375. |
| [68] | Mintzer M, Simanek E. Nonviral vectors for gene delivery[J]. Chemical Reviews, 2009, 109:259-302. |
| [69] | Ozcan G, Ozpolat B, Coleman R L, Sood A K, Lopez B G. Preclinical and clinical development of siRNA-based therapeutics[J]. Advanced Drug Delivery Reviews, 2015, 87: 108-119. |
| [70] | Neus F M, Esther V, Antonio V. Membrane-active peptides for non-viral gene therapy: making the safest easier[J]. Trends in Biotechnology, 2008, 26:267-275. |
| [71] | Mellert K, Lamla M, Scheffzek K, Wittig R, Kaufmann D. Enhancing endosomal escape of transduced proteins by photochemical internalisation[J]. Plos One, 2012, 7:52473-52473. |
| [72] | Jayakumar M K, Bansal A, Huang K, Yao R, Li B N, Zhang Y. Near-infrared-light-based nanoplatform boosts endosomal escape and controls gene knockdown in vivo[J]. ACS Nano, 2014, 8(5):4848-4858. |
| [73] | Wang X, Liu K, Yang G, Cheng L, He L, Liu Y, Li Y, Guo L, Liu Z. Near-infrared light triggered photodynamic therapy in combination with gene therapy using upconversion nanoparticles for effective cancer cell killing[J]. Nanoscale, 2014, 6:9198-9205. |
| [74] | Gu W Y,Jia Z F, Truong N P, Indira P, Yin X, Monteiro M J. Polymer nanocarrier system for endosome escape and timed release of siRNA with complete gene silencing and cell death in cancer cells[J]. Biomacromolecules, 2013, 14(10):3386-3389. |
| [75] | Juliette T, Jean M H L, Arthur S M, Te V, Jean P G. Past, present, and future of radiotherapy for the benefit of patients[J]. Nature Reviews Clinical Oncology, 2013, 10:52-60. |
| [76] | Hainfeld J F, Avraham D F, Slatkin D N, Smilowitz H M. Radiotherapy enhancement with gold nanoparticles[J]. Journal of Pharmacy & Pharmacology, 2008, 60:977-985. |
| [77] | Fan W P,Shen B,Bu W B,Chen F,He Q J,Zhao K L,Zhang S J,Zhou L P,Peng W J,Xiao Q F,Ni D L, Liu J N,Shi J L. A smart upconversion-based mesoporous silica nanotheranostic system for synergetic chemo-/radio-/photodynamic therapy and simultaneous MR/UCL imaging[J]. Biomaterials, 2014, 35(32):8992-9002. |
| [78] | Zhang C, Zhao K L, Bu W B, Ni D L, Liu Y Y, Feng J W, Shi J L. Marriage of scintillator and semiconductor for synchronous radiotherapy and deep photodynamic therapy with diminished oxygen dependence[J]. Angewandte Chemie, 2015, 54(6):1770-1774. |
| [79] | Lü R, Yang P, He F, Gai S, Li C, Dai Y, Yang G, Lin J. A yolk-like multifunctional platform for multimodal imaging and synergistic therapy triggered by a single near-infrared light[J]. ACS Nano, 2015, 9:1630-1647. |
| [80] | Jaque D, Maestro L M, Del R B, Haro G P, Benayas A, Plaza J, Rodr guez E M, Sol J G. Nanoparticles for photothermal therapies[J]. Nanoscale, 2014, 6:9494-9530. |
| [81] | Wang Y F, Liu G Y, Sun L D, Xiao J W, Zhou J C, Yan C H. Nd3+-sensitized upconversion nanophosphors: Efficient in vivo bioimaging probes with minimized heating effect[J]. ACS Nano, 2013, 7(8):7200-7206. |
| [82] | Zhan Q, Qian J, Liang H, Somesfalean G, Wang D, He S, Zhang Z, Andersson E S. Using 915 nm laser excited Tm3+/Er3+/Ho3+-doped NaYbF4 upconversion nanoparticles for in vitro and deeper in vivo bioimaging without overheating irradiation[J]. ACS Nano, 2011, 5(5):3744-3757. |
| [83] | Zou W, Visser C, Maduro J A, Pshenichnikov M S, Hummelen J C. Broadband dye-sensitized upconversion of near-infrared light[J]. Nature Photonics, 2012, 6:560-564. |
| [84] | Jayakumar M K, Idris N M, Huang K, Zhang Y. A paradigm shift in the excitation wavelength of upconversion nanoparticles[J]. Nanoscale, 2014, 6:8441-8443. |
| [85] | Sun Y, Feng W, Yang P, Huang C, Li F Y. The biosafety of lanthanide upconversion nanomaterials[J]. Chemical Society Reviews, 2015, 44:1509-1525. |
| [86] | Conner S D, Schmid S L. Regulated portals of entry into the cell[J]. Nature, 2003, 422:37-44. |
| [87] | Durymanov M O, Rosenkranz A A, Sobolev A S. Current approaches for improving intratumoral accumulation and distribution of nanomedicines[J]. Theranostics, 2015, 5:1007-1020. |
| [88] | Jin J, Gu Y J, Man C W Y, Cheng J, Xu Z, Zhang Y, Wang H, Lee V H Y, Cheng S H, Wong W T. Polymer-coated NaYF4:Yb3+, Er3+ upconversion nanoparticles for charge-dependent cellular imaging[J]. ACS Nano, 2011, 5(10):7838-7847. |
| [89] | Liu C, Hou Y, Gao M. Are rare-earth nanoparticles suitable for in vivo applications[J]. Advanced Materials, 2014, 26:6922-6932. |
| [90] | Gnach A, Lipinski T, Bednarkiewicz A, Rybka J, CapobiancoJA. Upconverting nanoparticles: assessing the toxicity[J]. Chemical Society Reviews, 2015, 44:1561-1584. |
| [91] | Chen Y M, Jiang G Y, Zhou Q X, Zhang Y Y, Li K, Zheng Y, Zhang B W, Wang X S. An upconversion nano-particle/Ru(Ⅱ) polypyridyl complex assembly for NIR-activated release of a DNA covalent-binding agent[J]. RSC Advances, 2016, 6(28):23804-23808. |





