2. College of Chemistry and Material Science, Hebei Normal University, Shijiazhuang 050024, Hebei, P. R. China;
3. Key Laboratory of Pesticide and Chemical Biology, Ministry of Education, College of Chemistry, Central China Normal University, Wuhan 430079, Hubei, P. R. China
2. 河北师范大学 化学与材料学院, 河北 石家庄 050024;
3. 华中师范大学 化学学院 农药与化学生物学教育部重点实验室, 湖北 武汉 430079
In recent years, the development of chemical sensors for heavy metals has attracted an increasing attention owing to their bioaccumulation, biomagnification, and persistence in the environment and health[1-4]. Due to the thiophilic nature in proteins and enzymes of mercury ion (Ⅱ), mercury ion (Ⅱ) has been one of the most toxic and dangerous heavy metal ions[5, 6]. The exposure of body to excessive amounts of mercury ion (Ⅱ) can lead to dysfunctions of the cells, brain, kidney, stomach, even central nervous system[7, 8]. Therefore, it is very significative to develop a simple method to determine Hg2+ efficiently.
In the last few years, great attention has been paid to the development of organic small molecules based fluorescent chemosensors due to its high sensitivity, selectivity, and relatively simple synthesis[9-12]. Over the past decades, many mercury ion (Ⅱ) fluorescent probes with high selectivity have been widely developed and applied in bioimaging[13-18].According to these reports, it was found that the determining mechanism of these mercury ion (Ⅱ) probes mainly involved in two strategies. One is about the direct desulfurization reaction of mercury ion (Ⅱ) with the thiourea group on the probe molecule[19-21]. The other is based on the coordination bond formation between the probe molecule and mercury ion (Ⅱ) that is considered as the most common approaches[9-12].Accordingly, all kinds of metal ligands were introduced to the fluorescent dyes to construct the selective fluorescent probes for the detection of mercury ion (Ⅱ). For example, morpholine group containing a nitrogen atom and an oxygen atom can be used as the metal ligand to construct the fluorescent probes for the detection of metal cations. For instance, Westmeyer et al employed the morpholine group as a ligand unit to develop a cyanine-based Ca2+-responsive fluorescent probe[22].Recently, we also introduced morpholine group to the fluorescent dye 4-chloro-7-nitrobenzo-2, 1, 3-oxadiazole (NBD-Cl), constructing a highly selective Hg2+-responsive fluorescent probe, which could be used to visualize the level of mercury ion (Ⅱ) in living cells[23].
In view of the complicated synthesis of Hg2+-responsive fluorescent probes, based on our recent works[24-35], we designed and synthesized a highly selective Hg2+-responsive fluorescent probe by using a dansyl fluorescent dye in this research. The result showed that this probe had good water-solubility, membrane permeability and biocompatibility, and could be applied in the bioimaging of mercury ion (Ⅱ) in living cells.
2 Experiment 2.1 GeneralAll reactions were taken place by using standard Schlenk techniques under an argon atmosphere, unless elaborated. All reagents and materials were purchased commercially and without further purification. Dansyl chloride (97%) was obtained by commercial approach. The 4-(2-aminoethyl) morpholine was synthesized according to our work[8].CH2Cl2 was dried with CaH2, and then distilled under nitrogen atmosphere. Column chromatography was performed over silica gel (200-300 mesh). NMR spectra were obtained on American Varian Mercury Plus 600 spectrometer (600 MHz) or 400 MHz and their chemical shifts are relative to TMS. Electrospray (EI) mass spectra were carried on Firmigan Trace. UV-Vis spectra were obtained on U-3310 UV Spectrophotometer. Fluorescence spectra were taken on a Fluoromax-P luminescence spectrometer.
2.2 Synthesis of probe 1To a 50 mL round-bottomed flask was added 122 mg (1 mmol) of 2-morpholinoethanamine and 20 mL of anhydrous CH2Cl2. Then triethylamine (0.2 mL) was added to the mixture. 296.5 mg (1.1 mmol) of 5-dimethylaminonaphthalene-1-sulfonyl chloride was added dropwise under stirring at 0 ℃. The reaction was stirred at room temperature for 4 hours. The mixture was then evaporated and the residue was purified with column chromatography (silica gel, dichloromethane/ethyl acetate=10/1, V/V). 0.312 g of yellow solid was obtained, yield: 86 %.
1HNMR (400 MHz, CDCl3): 8.52-8.50 (d, J=8.00 Hz, 1H), 8.32-8.29 (d, J=12.00Hz, 1H), 8.25-8.23(d, J=8.00 Hz, 1H), 7.59-7.55 (t, J=8.00 Hz, 1H), 7.54-7.50 (t, J=8.00 Hz, 1H), 7.18-7.16 (d, J=8.00 Hz, 1H), 5.55 (s, 1H), 3.31(s, 4H), 2.88-2.81(m, 8H), 2.21-2.18 (t, 2H), 1.95-1.94 (t, 8.00 Hz, 2H). 13CNMR (CDCl3, 400 MHz): 152.0, 134.2, 130.2, 129.6, 129.4, 128.3, 123.1, 118.5, 115.1, 77.6, 77.3, 77.0, 66.3, 55.6, 52.5, 45.3, 39.2. EI-MS m/z(M+) calcd 363.16, found 363.29.
2.3 Optical studies of probe 1 upon addition of various analytesA stock solution of probe 1 (1 mmol/L) was prepared in DMSO, and a stock solution of metal ions (10 mmol/L) were dissolved in distilled water. We selected a wide range of metal ions, such as Ca2+, Ni2+, Li+, Al3+, Cd2+, K+, Ag+, Zn2+, Pb2+, Mn2+, Cu2+, Fe3+, Na+, Fe2+ and Hg2+. For selectivity, the work solution of probe 1 (10 μmol/L) was prepared by mixing 1 mmol/L of probe 1 and 0.1 mmol/L of metal ions in HEPES buffer solution (pH=7.4, 0.02 mol/L) containing 5% DMSO solution. For titration, the work solution of probe 1 (10 μmol/L) was obtained by mixing 1 mmol/L of probe 1 in HEPES buffer solution (pH=7.4, 0.02 mol/L) containing 5% DMSO solution with varying concentration of Hg2+ from 0 to 700 μmol/L. For all fluorescence spectra were collected from 450 to 700 nm excitation with 380 nm.
2.4 Cell culture and imagingThe human cervical carcinoma cell lines Hela was obtained from American Type Culture Collection (ATCC, USA). The cell lines were cultured in DMEM medium supplemented with 10% (V/V) calf serum, penicillin (100 U/mL) and (100 mg/mL) streptomycin. Cells were maintained at 37 ℃ in a humidified atmosphere containing 5% CO2. The cells were seeded in laser confocal fluorescence microscope (LCFM) culture dishes with a density of 5×105 cells/well. The cells lines were cultured in DMEM medium supplemented with 10% (V/V) calf serum, penicillin (100 U/mL) and streptomycin (100 mg/mL). Cells were maintained at 37 ℃ in a humidified atmosphere containing 5% CO2. When the whole cells took up 70%-80% space of culture dishes, two groups were studied as followed. The cells were firstly maintained at 37 ℃ for 0.5 h after introduction of probe 1 (100 μL, 10 μg/mL) and then treated with Hg2+ (100 μL, 1 mg/mL) for 0.5 h. The medium was then carefully removed and each dish was washed three times with PBS (pH=7.0). Then the fluorescence of probe 1 was collected by LCFM (FV 1000, Olympus, Japan). To study the cytotoxicity, cells were seeded at 1×105 cells per well in 24-well plates and incubated for 24 h before treatment, followed by exposure to different concentrations (2-50 μmol/L) of probe 1 for an additional 24 h. To determine cell viability, the colorimetric metabolic activity assay with 3-(4, 5-dimethylthiazol-2-yl)-3, 5-diphenytetrazo-lium bromide (MTT) was used. Cell samples then were incubated with MTT solution (containing 5 mg/mL MTT reagent in PBS) for 4 h, and after removal of the MTT solution, dimethyl sulfoxide was added to dissolve the formazan crystals. The absorbance was measured at 540 nm with a microplate reader. Each experiment was performed at least three times. The cytotoxic effect of the probe was assessed through the ratio of the absorbance of the probe treated sample versus the control sample.
3 Results and discussion 3.1 Synthesis and characterizationThe general synthesis of probe 1 was outlined in Scheme 1. The commercial fluorescent dye dansyl chloride 2 was treated with 4-(2-aminoethyl) morpholine 3 in the presence of trimethylamine at room temperature to give the targeting probe 1 in 86% yield. The probe 1 was well characterized by 1HNMR, 13CNMR and mass spectroscopy (MS).

|
Scheme1 Synthesis of probe 1 |
Firstly, the basic optical properties of probe 1 was investigated. Probe 1 showed a strong fluorescence at 555 nm upon the irradiation of 380 nm light in HEPES buffer solution (pH=7.4, 0.02 mol/L) containing 5% DMSO solution. To evaluate the selectivity of probe 1 in a mixed solution of DMSO-HEPES (V/V=5/95, pH=7.4, 0.02 mol/L), many metal ions were investigated such as Ca2+, Ni2+, Li+, Al3+, Cd2+, K+, Ag+, Zn2+, Pb2+, Mn2+, Cu2+, Fe3+, Na+, Fe2+ and Hg2+. As shown in Figure 1A and 1B, the addition of Hg2+ (10 equiv.) induced a fluorescence change of probe 1 (10 μmol/L) along with an obvious fluorescence change in Figure 1C. However, no obvious changes in the fluorescence spectra of probe 1 was observed when other metal ions (10 equiv.) were added to the solution of probe 1. The result suggested that probe 1 had a good water-solubility and could highly selective detect the mercury ion (Ⅱ).
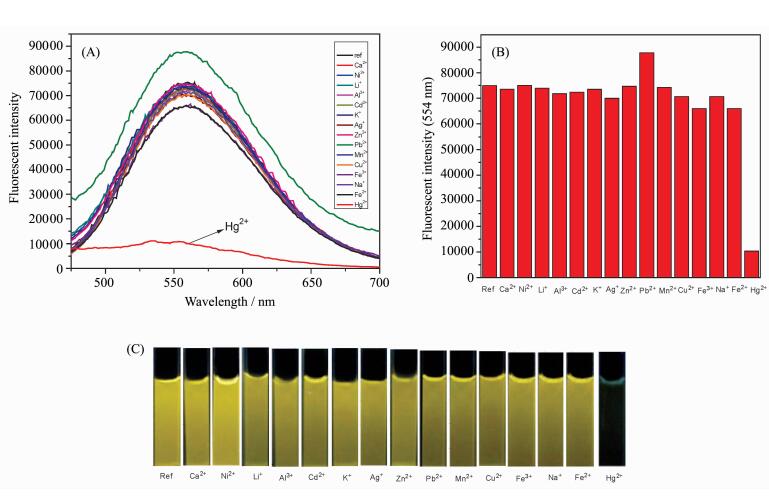
|
Fig.1 (A) Fluorescence spectra and (B) fluorescence intensity at 555 nm of probe 1 (10 μmol/L) in the presence of different metal ions (10 equiv) such as Ca2+, Ni2+, Li+, Al3+, Cd2+, K+, Ag+, Zn2+, Pb2+, Mn2+, Cu2+, Fe3+, Na+, Fe2+ and Hg2+ in HEPES buffer solution (pH=7.4, 0.02 mol/L) containing 5% DMSO solution; (C) Fluorescence changes of probe 1 Left to right: 1 only (ref.), Ca2+, Ni2+, Li+, Al3+, Cd2+, K+, Ag+, Zn2+, Pb2+, Mn2+, Cu2+, Fe3+, Na+, Fe2+ and Hg2+ (λex=380 nm) |
To give a further insight into the high selectivity of probe 1 (10 μmol/L) toward Hg2+, the fluorescence interference experiment of other various metal ions was also investigated. Hg2+ (10 equiv.) was added to probe 1 (10 μmol/L) in HEPES buffer solution (pH=7.4, 0.02 mol/L) containing 5% DMSO solution, then other metal ions (10 equiv.) such as Ca2+, Ni2+, Li+, Al3+, Cd2+, K+, Ag+, Zn2+, Pb2+, Mn2+, Cu2+, Fe3+, Na+, Fe2+ and Hg2+ were also added into the solution, respectively. Their fluorescence intensities were recorded in Figure 2. The fluorescence intensity of 1-Hg2+ in DMSO-HEPES (V/V=5/95, pH=7.4, 0.02 mol/L) solution containing other metal ions did not show any significant changes in comparison with the fluorescence intensity of 1-Hg2+ without other metal ions. All the results indicated that the probe 1 could be used as a highly selective fluorescent probe for Hg2+.
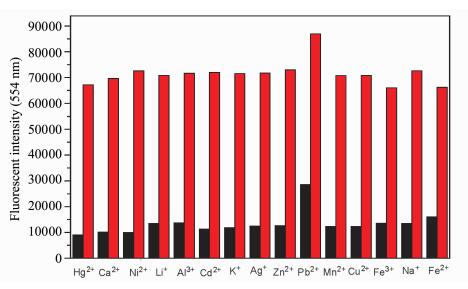
|
Fig.2 Fluorescence response of probe 1 (10 μmol/L) to Hg2+, Ca2+, Ni2+, Li+, Al3+, Cd2+, K+, Ag+, Zn2+, Pb2+, Mn2+, Cu2+, Fe3+, Na+ and Fe2+ (10 equiv) in HEPES buffer solution (pH=7.4, 0.02 mol/L) containing 5% DMSO solution Red bar: probe 1 + other metal ions. Black bar: probe 1 + other metal ions +Hg2+ (λex= 380 nm) |
To obtain detailed information about the sensitivity of probe 1, the fluorescence intensity changes were monitored upon the addition of various concentrations of Hg2+ into the probe. Accordingly, the titration experiment of probe 1 (10 μmol/L) towards Hg2+ in HEPES buffer solution (pH=7.4, 0.02 mol/L) containing 5% DMSO solution was performed. The result of the titration experiment investigating the Hg2+ concentration-dependent behavior displayed that the fluorescence intensity of the emission peak at 555 nm decreased gradually with the increase of Hg2+ concentration, as presented in Figure 3A and 3B. Moreover, a good linear relationship was observed with the concentration of Hg2+ from 0 to 20 μmol/L in Figure 3D. It was worth mentioning that the detection limit of probe 1 to Hg2+ was calculated to be 2.1×10-8 mol/L according to a further titration in Figure 3C. In comparison to some reports[13-18, 23], probe 1 had a lower detection limit. These results described above strongly implied that probe 1 could be used as a fluorescence indicator to visualize the level of mercury ion (Ⅱ) in living cells. In order to understand the binding mode between probe 1 and Hg2+, Job's plot analysis was carried out. As shown in Figure 4, it was found that probe 1 could form a 1:1 stoichiometrical complex with Hg2+.
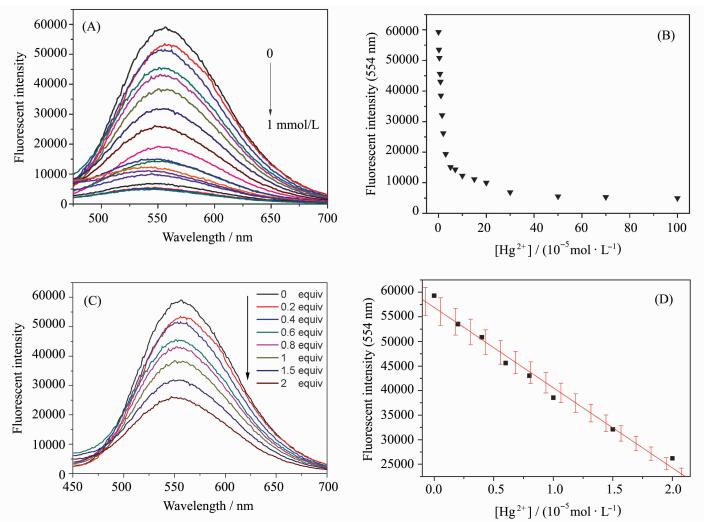
|
Fig.3 Fluorescence spectra of probe 1 (10 μmol/L) upon addition of Hg2+ (0-1 mmol/L for A and 0-20 μmol/L for C) in HEPES buffer solution (pH=7.4, 0.02 mol/L) containing 5% DMSO solution and the corresponding relationship between the fluorescence intensity and Hg2+ concentrations (0-1 mmol/L for B and 0-20 μmol/L for D) (λex=380 nm) |
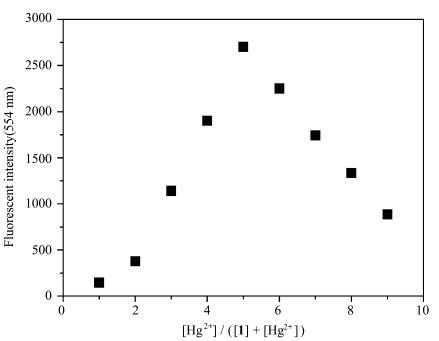
|
Fig.4 Job's plot of probe 1 and Hg2+ ([1] + [Hg2+] = 10 μmol/L) in HEPES buffer solution (pH=7.4, 0.02 mol/L) containing 5% DMSO solution, λex=380 nm |
As described above, probe 1 showed good water-solubility and high selectivity toward Hg2+without interferences from other metal cations and with low detection limit, which supported it to monitor toxic Hg2+ in cell levels. To achieve this purpose, HeLa cells were cultured in DMEM supplemented with 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, and 100 mg/mL streptomycin and were maintained in an incubator at 37 ℃ in a 5% CO2/air environment. Probe 1 (100 μmol/L) was incubated with HeLa cells in DMEM for 30 min at 37 ℃, and then the HeLa cells were washed with PBS. As presented by bright field and merged images displayed in Figure 5A-5C, a green fluorescence was produced in the cytoplasm of HeLa cells. The results indicate that probe 1 was readily internalized into living cells and had excellent cell permeability and had lower toxicity (In Figure S1). However, an obvious weaken fluorescence was detected when HeLa cells were incubated with probe 1 for 30 min and then treated with Hg2+ for additional 30 min in Figure 5D. As a result, probe 1 could be employed as an efficient tool to monitor the level of Hg2+ in living cells.

|
Fig.5 Fluorescence microscopy images of cells incubated with probe 1 (100 μmol/L) in DMEM before and after addition of Hg2+ (1 mmol/L) (A) bright field image after addition probe 1; (B) fluorescence after addition probe 1; (C) overlap of A and B; (D) fluorescence after addition probe 1 and treatment with Hg2+ |
A new fluorescent probe based on dansyl fluorescent dye with good water-solubility, high selectivity and good sensitivity toward Hg2+ was designed and synthesized. And this probe displayed a very low detection limit upon to 2.1×10-8 mol/L. The bioimaging showed that this probe could be used to visualize the level of mercury ion (Ⅱ) in living cells. Furthermore, it also confirmed that this probe had good membrane permeability and biocompatibility. We believed that it could act as an efficient tool to monitor the mercury ion (Ⅱ) in living cells.
Acknowledgements The authors acknowledge financial support from National Natural Science Foundation of China (21676113, 21402057, 21472059); Youth Chen-Guang Project of Wuhan (2016070204010098). Supported by the Ministry-Province Jointly Constructed Base for State Key Lab-Shenzhen Key Laboratory of Chemical Biology, Shenzhen; Financially supported by self-determined research funds of CCNU from the college' basic research and operation of MOE (CCNU16A02004).| [1] | Leray I, Valeur B. Calixarene-based fluorescent molecular sensors for toxic metals[J]. European Journal Inorganic Chemistry, 2009, 24: 3525–3535. |
| [2] | Zhao Q, Li F, Huang C. Phosphorescent chemosensors based on heavy-metal complexes[J]. Chemical Society Review, 2010, 39(8): 3007–3030. DOI:10.1039/b915340c |
| [3] | Sun W H D, Wu Z, Song B, Yang S. Research progress of fluorescent molecular probes for heavy and transition metallic cations based on rhodamine fluorophore[J]. Chinese Journal of Organic Chemistry, 2011, 31(8): 997–1010. |
| [4] | Zhu G, Zhang C. Functional nucleic acid-based sensors for heavy metal ion assays[J]. Analyst, 2014, 139(24): 6326–6342. DOI:10.1039/C4AN01069H |
| [5] | Clarkson T W, Magos L, Myers G J. The toxicology of mercury-current exposures and clinical manifestations[J]. The New England Journal of Medicine, 2003, 349: 1731–1737. DOI:10.1056/NEJMra022471 |
| [6] | Kosnett M J. Chelation for heavy metals (arsenic, lead, and mercury):protective or perilous?[J]. Clinical Pharmacology & Therapeutics, 2010, 88(3): 412–415. |
| [7] | Von Burg R. Toxicology update[J]. Journal of Applied Toxicology, 1995, 15(6): 483–493. DOI:10.1002/(ISSN)1099-1263 |
| [8] | Harris H H, Pickering I J, George G N. The chemical form of mercury in fish[J]. Science, 2003, 301(5637): 1203–1203. DOI:10.1126/science.1085941 |
| [9] | Yin J, Hu Y, Yoon J. Fluorescent probes and bioimaging: alkali metals, alkaline earth metals and pH[J]. Chemical Society Review, 2015, 44(14). |
| [10] | Zhu H, Fan J, Wang B, Peng X. Fluorescent, MRI, and colorimetric chemical sensors for the first-row d-block metal ions[J]. Chemical Society Reviews, 2015, 44(13): 4337–4366. DOI:10.1039/C4CS00285G |
| [11] | Wu X, Zhu W. Stability enhancement of fluorophores for lighting up practical application in bioimaging[J]. Chemical Society Reviews, 2015, 44(13): 4179–4184. DOI:10.1039/C4CS00152D |
| [12] | Yang Y, Zhao Q, Feng W, Li F. Luminescent chemodosimeters for bioimaging[J]. Chemical Reviews, 2013, 113(1): 192–270. DOI:10.1021/cr2004103 |
| [13] | Nolan E M, Lippard S J. Tools and tactics for the optical detection of mercuric ion[J]. Chemical Reviews, 2008, 108(9): 3443–3480. DOI:10.1021/cr068000q |
| [14] | Ono A, Torigoe H, Tanaka Y, Okamoto I. Binding of metal ions by pyrimidine base pairs in DNA duplexes[J]. Chemical Society Reviews, 2011, 40(12): 5855–5866. DOI:10.1039/c1cs15149e |
| [15] | Kim H N, Ren W X, Kim J S, Yoon J. Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions[J]. Chemical Society Reviews, 2012, 41(8): 3210–3244. DOI:10.1039/C1CS15245A |
| [16] | Culzoni M J, de la Peña A M, Machuca A, Goicoechea H C, Babiano R. Front cover[J]. Analytical Methods, 2013, 5: 30–49. DOI:10.1039/C2AY25769F |
| [17] | Kumar N, Bhalla V, Kumar M. Resonance energy transfer-based fluorescent probes for Hg2+, Cu2+ and Fe2+/Fe3+ ions[J]. Analyst, 2014, 139(3): 543–558. DOI:10.1039/C3AN01896B |
| [18] | Chen G, Guo Z, Zeng G, Tang L. Fluorescent and colorimetric sensors for environmental mercury detection[J]. Analyst, 2015, 140(16): 5400–5443. DOI:10.1039/C5AN00389J |
| [19] | Zhang X, Xiao Y, Qian X. A ratiometric fluorescent probe based on FRET for imaging Hg2+ ions in living cells[J]. Angewandte Chemie International Edition, 2008, 47(42): 8025–8029. DOI:10.1002/anie.v47:42 |
| [20] | Guo Z, Zhu W, Zhu M, Wu X, Tian H. Near-infrared cell-permeable Hg2+-selective ratiometric fluorescent chemodosimeters and fast indicator paper for MeHg+ based on tricarbocyanines[J]. Chemistry-A European Journal, 2010, 16(48): 14424–14432. DOI:10.1002/chem.201001769 |
| [21] | Liu Y, Chen M, Cao T, Sun Y, Li C, Liu Q, Yang T, Yao L, Feng W, Li F. A cyanine-modified nanosystem for in vivo upconversion luminescence bioimaging of methylmercury[J]. Journal of the American Chemical Society, 2013, 135(26): 9869–9876. DOI:10.1021/ja403798m |
| [22] | Mishra A, Jiang Y, Roberts S, Ntziachristos V, Westmeyer G G. Near-infrared photoacoustic imaging probe responsive to calcium[J]. Analytical Chemistry, 2016, 88(22): 10785–10789. DOI:10.1021/acs.analchem.6b03039 |
| [23] | Zhang Y, Chen H, Chen D, Wu D, Chen Z, Zhang J, Chen X, Liu S H, Yin J. A colorimetric and ratiometric fluorescent probe for mercury (Ⅱ) in lysosome[J]. Sensors and Actuators B: Chemical, 2016, 224: 907–914. DOI:10.1016/j.snb.2015.11.018 |
| [24] | Yin J, Kwon Y, Kim D, Lee D, Kim G, Hu Y, Ryu J-H, Yoon J. Cyanine-based fluorescent probe for highly selective detection of glutathione in cell cultures and live mouse tissues[J]. Journal of American Chemical Society, 2014, 136(14): 5351–5358. DOI:10.1021/ja412628z |
| [25] | Kim D, Kim G, Nam S J, Yin J, Yoon J. Elaborately tuning intramolecular electron transfer through varying oligoacene linkers in the bis (diarylamino) systems[J]. Scientific Reports, 2015, 5: 8488. DOI:10.1038/srep08488 |
| [26] | Cao M, Jiang L, Hu F, Zhang Y, Yang W C, Liu S H, Yin J. A dansyl-based fluorescent probe for selectively detecting Cu2+ and imaging in living cells[J]. RSC Advances, 2015, 5(30): 23666–23670. DOI:10.1039/C5RA00740B |
| [27] | Lee D, Kim G, Yin J, Yoon J. An aryl-thioether substituted nitrobenzothiadiazole probe for the selective detection of cysteine and homocysteine[J]. Chemical Communications, 2015, 51(30): 6518–6520. DOI:10.1039/C5CC01071C |
| [28] | Hu F, Cao M, Huang J, Chen Z, Wu D, Xu Z, Liu S. H, Yin J. Sulfonamide and urea-based anions chemosensors[J]. Dyes and Pigments, 2015, 119: 108–115. DOI:10.1016/j.dyepig.2015.03.036 |
| [29] | Hu F, Cao M, Ma X, Liu S.H, Yin J. Visible-light-dependent photocyclization: design, synthesis, and properties of a cyanine-based dithienylethene[J]. The Journal of Organic Chemistry, 2015, 80(15): 7830–7835. DOI:10.1021/acs.joc.5b01466 |
| [30] | Yin J, Kwon Y, Kim D, Lee D, Kim G, Hu Y, Ryu J H, Yoon J. Preparation of a cyanine-based fluorescent probe for highly selective detection of glutathione and its use in living cells and tissues of mice[J]. Nature Protocols, 2015, 10: 1742–1754. DOI:10.1038/nprot.2015.109 |
| [31] | Zhang Y, Chen H, Chen D, Wu D, Chen X, Liu S H, Yin J. A fluorescent turn-on H2S-responsive probe: design, synthesis and application[J]. Organic & Biomolecular Chemistry, 2015, 13(38): 9760–9766. |
| [32] | Cao M, Chen H, Chen D, Xu Z, Liu S H, Chen X, Yin J. Naphthalimide-based fluorescent probe for selectively and specifically detecting glutathione in the lysosomes of living cells[J]. Chemical Communications, 2016, 52(4): 721–724. DOI:10.1039/C5CC08328A |
| [33] | Sheng X, Chen D, Cao M, Zhang Y, Han X, Chen X, Liu S H, Chen H, Yin J. A near infrared cyanine-based fluorescent probe for highly selectively detecting glutathione in living cells[J]. Chinese Journal of Chemistry, 2016, 34: 594–598. DOI:10.1002/cjoc.v34.6 |
| [34] | Sheng X, Ye Z, Liu S H, He W, Ren J, Yin J. Cyanine IR-780 for distinguishing 2-amino thiophenols from position isomers[J]. Dyes and Pigments, 2016, 131: 84–90. DOI:10.1016/j.dyepig.2016.03.045 |
| [35] | Hu Y, Yin J, Yoon J. A multi-responsive cyanine-based colorimetric chemosensor containing dipicolylamine moieties for the detection of Zn (Ⅱ) and Cu (Ⅱ) ions[J]. Sensors and Actuators B: Chemical, 2016, 230: 40–45. DOI:10.1016/j.snb.2016.02.040 |




