Research into carbon nanostructures has opened the door to new possibilities in engineering and nanotechnology. As more applications are found, it has pushed scientists to look for the next generation of inexpensive, versatile materials. One element that has become of great interest in the last few years is phosphorus. With its different stable and semi-stable allotropes, phosphorus provides unique potential for an array of applications due to its electronic and, specifically, optical properties.
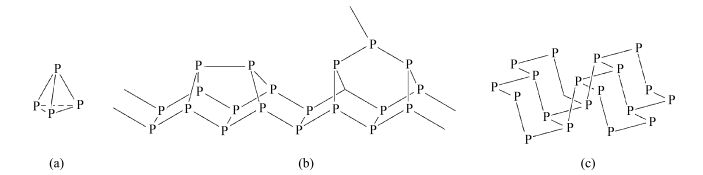
There are three common allotropes of phosphorus: white phosphorus, red phosphorus, and black phosphorus [1]. White phosphorus exists as molecular P4, with strained P—P bonds. This causes white phosphorus to be extremely unstable and will ignite upon contact with air to form phosphorus oxides [2]. Red phosphorus is a stable, non-toxic solid produced by heating white phosphorus between 200 ℃ and 250 ℃ in a vacuum [1, 2]. Red phosphorus exists in a number of different forms, both crystalline andamorphous, with Type Ⅳ being called fibrous red phosphorus and Type Ⅴ being known as Hittorf's phosphorus or violet phosphorus [2, 3]. Black phosphorus is the thermodynamically stable allotrope most widely studied for its semiconducting properties [2, 4]. 2D sheets of black phosphorus have been termed phosphorene, which can be produced by chemical vapor deposition of red phosphorus at 600 ℃ [4]. Structures for the solid phosphorus allotropes are detailed in Figure 1.
|
Fig.1 Molecular structures of common allotropes of phosphorus (a) white phosphorus, P4, (b) type Ⅳ and Ⅴ red phosphorus, (c) black or 2D phosphorus (phosphorene) |
Although studies on phosphorus nanostru-ctures and properties are still in their infancy, unique properties of phosphorus nanostructure have been observed. In this short review, we intend to summarize a photochemical properties, optical and optoelectronic applications of phosphorus. Prospective research of these materials is also discussed.
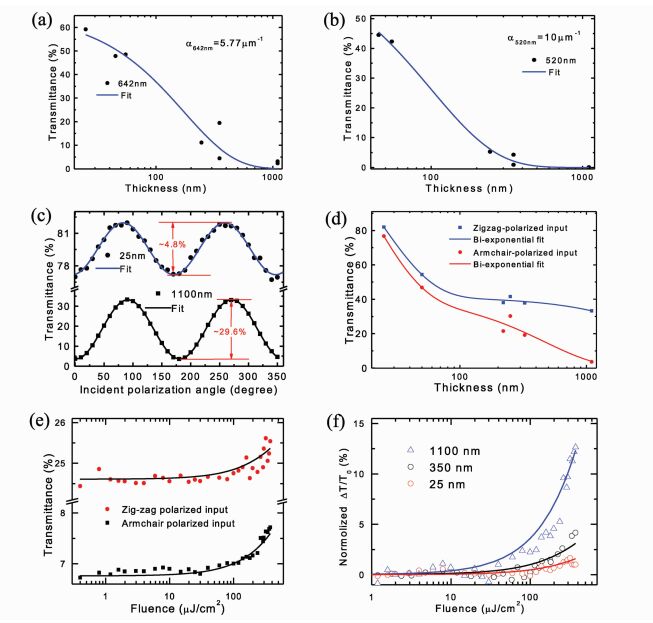
2 Nonlinear Properties of Black PhosphorusBlack phosphorus (BP) flakes have been studied for their nonlinear properties. Li et al. [5] observed that both the linear and nonlinear optical properties are anisotropic and can be tuned by the black phosphorus film thickness, which is not observed in other 2D layered materials, such as graphene and most transition metal dichalcogenides. The team utilized the nonlinear properties for an ultrafast high energy pulse study. The pulse duration is reported at approximately 786 fs in mode-locking and the pulse energy's maximum output is about 18.6 nJ in Q-switching. The black phosphorus film thickness was measured by Atomic Force Microscopy (AFM). The results of transmittance of the black phosphorus thin films versus thickness of the films is shown in Figure 2.
|
Fig.2 Linear and nonlinear optical properties of BP films: Transmittance of BP films as a function of thickness at the wavelengths of (a) 642 nm and (b) 520 nm. (c) Polarization dependent transmittance for 25 nm and 1100 nm thick BP films. (d) Transmittance of BP films as a function of film thickness at the wavelength of 1550 nm with two orthogonal polarized light directions. (e) Fluence dependent transmittance of the 1100 nm thick BP film measured with ultrafast pulses at two orthogonal polarized light directions. (f) Relative transmittance change measured from 25 nm, 350 nm and 1100 nm thick BP films as a function of input pulse fluence. The input polarization direction is along the armchair direction of the BP films. Reproduced with permission under Creative Commons License from reference [5]. |
The polarization directions that correspond to the maximum and minimum of the transmittance curves in Figure 2(c) are linked with the zigzag and armchair axes of the black phosphorus thin films [5]. Specifically, Figures 2(e) and 2(f) correspond to the nonlinear properties of black phosphorus thin films. The thin films were transferred onto the ends of optical fibers to build the pulsed fiber laser used in the paper. Q-switched optical output from the fiber laser was achieved only after inserting the BP integrated device inside the approximately 11 m cavity [5]. Q-switching operation was achieved by the group with all black phosphorus samples, with the 1100 nm thick thin film giving the better performance (the relatively large transmittance change performance in the device from Figure 2(f) demonstrates this) [5].
The authors increased the cavity from around 11 m to about 14.2 m after adding about 3 meters of SMF-28 single mode fiber in the laser cavity. After inserting the BP integrated fiber device into the fiber cavity, stable mode-locking was initiated by introducing a disturbance to the intra-cavity fiber [5].
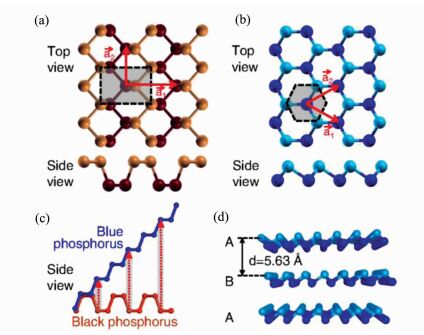
3 Optical Reflectance of PhosphorusIn 2015, Hu T, Hashmi A, and Hong J conducted a theoretical study on the geometry, electronic structures, and optical properties of phosphorus nanotubes (PNTs) [6]. The basis for their work came from a paper published only a year prior to theirs in which Zhu Z and Tománek D conducted a computational study on a currently unrecognized allotrope of phosphorus, which they dubbed 'blue phosphorus' [7]. Structural differences between black phosphorus and blue phosphorus can be seen in Figure 3.
|
Fig.3 The layered structure of (a) black and (b) blue phosphorus in top and side views. Atoms at the top and bottom of the non-planar layers are distinguished by color and shading, and the Wigner-Seitz cells are shown by the shaded region. (c) Schematic of the conversion of black to blue phosphorus by dislocations, highlighted by the shaded regions and arrows. (d) Equilibrium structure of AB stacked blue phosphorus in side view. Reprinted Figure with permission from reference [7]. Copyright 2014 by the American Physical Society. |
Hu et al. [6] investigated two distinct types of phosphorus nanotubes, which they named α-PNTs for the blue phosphorus monolayers and β-PNTs for the black phosphorus monolayers. The team predicts that both armchair and zigzag geometries (analogous to carbon nanotubes) are possible for α-PNTs, but only the armchair geometry is feasible with β-PNTs because the zigzag conformation induces too much strain from conformational energies.
The band structures revealed that the band gap is larger for the blue phosphorus α-PNTs than the band gap for the black phosphorus β-PNTs. The researchers found that the band gap for α-PNTs did not depend on the chirality (armchair or zigzag) of the phosphorus tubule, nor the inner diameter of the tubule when the inner diameter exceeds 1.3 nm, but the β-PNTs do in fact show chirality and diameter dependence [6].
This all led to the researchers discussing the optical properties. Hu et al. [6] presented the frequency-dependent dielectric function (see Equation 1) for the armchair and zigzag α-PNTs and the armchair configuration for the β-PNTs and considered parallel electric fields (E||) and perpendicular electric fields (E⊥) with respect to the nanotube axis.
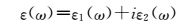
|
(1) |
They noted no significant disparity in the frequency-dependent dielectric functions among armchair nanotubes with different indices in α-PNTs, and the zigzag α-PNTs show the same trend [6]. They also discovered that the magnitude of the dielectric function for parallel polarization is generally larger than that for perpendicular polarization, which the authors hypothesize will result in asymmetric optical properties. For the blue phosphorus α-PNT armchair nanotubes, ε1(0) was 2.7 for parallel polarization and 2 for perpendicular polarization. For the α-PNT zigzag nanotubes, ε1(0) was 1.7 for parallel polarization and 1.4 for perpendicular polarization. This demonstrates a directional dependence with the α-PNTs on the angle of incoming polarized light. Interestingly, the β-PNTs were shown to be independent of the polarization direction, with a value of 2.9 for parallel polarization and 2.8 for perpendicular polarization [6].
The researchers graphed the reflectivity of different phosphorus nanotubes using the following equation [6]:

|
(2) |
where the epsilon functions come from Equation 1. The graphs are shown in Figure 4.
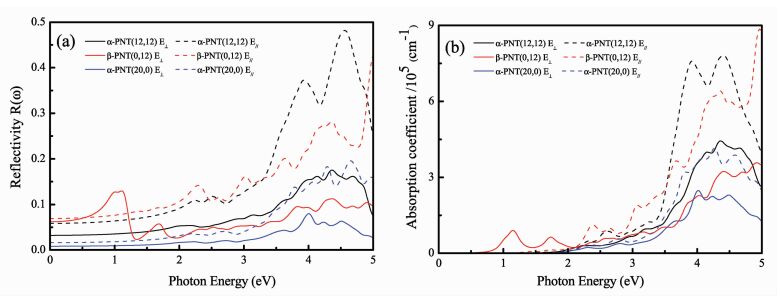
|
Fig.4 Calculated optical quantities: (a) reflectivity (b) absorptioncoefficients of α-PNTs (12, 12), (20, 0) and β-PNTs (0, 12) © IOP Publishing. Reproduced with permission from reference [6] |
For the blue phosphorus α-PNTs and black phosphorus β-PNT (the armchair configuration), the phosphorus nanotubes had greater reflectivity for the parallel polarization than for the perpendicular polarization, but the zigzag α-PNT form showed very weak reflectivity in the visible frequencies, demonstrating strong asymmetric behavior regarding the polarization direction [6]. Hu et al. [6] hypothesized that this weak reflectivity value might cause issues for reflecting or anti-reflecting applications.
While not referenced in the Hu et al. paper, there was research published in 1984 from Asahina H and Morita A that worked with many of the same topics [8]. Asahina and Morita's paper go into the optical properties of black phosphorus single crystals as opposed to phosphorus nanotubes. The pair also utilized the frequency-dependent dielectric function from Equation 1 some 30 years before the Hu et al paper. However, it is their research into the reflective properties of black phosphorus that drew the most interest for this review.
Asahina and Morita cited a 1983 paper by themselves with Maruyama Y where they measured the reflectance spectra on the cleaved xy-surface of a black phosphorus single crystal at room temperature for linearly polarized light in the range from 400 to 2000 nm [8]. The results from the 1983 paper are shown via solid lines in Figure 5 and another paper (Taniguchi et al. (1982)) is mentioned, but are not independently cited since they are presented in the 1984 paper and are not being taken or mentioned directly from these works.

|
Fig.5 Reflectance spectra in the region from visible to vacuum ultraviolet
(a) Experimental curves obtained by Taniguchi et al. (1982) and (b) theoretical ones. Full curves are for the polarisation 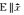 and broken curves for the polarisation and broken curves for the polarisation  . © IOP Publishing. Reproduced with permission from reference [8] . © IOP Publishing. Reproduced with permission from reference [8]
|
To keep consistent with Figure 4, the x-axis units in Figure 5 for photon energy, Ryd, can be converted to the electronvolts units using the NIST conversion from Rydbergs to eV (1 Ryd = 13.605693009(84) eV) [9], converting the bottom axis from 0, 0.5, 1.0 and 1.5 Ryd to 0, 6.8, 13.6 and 20.4 eV, respectively. This demonstrates that different forms of phosphorus exhibit varying degrees of reflectance at different photon energies, which makes phosphorus a versatile material depending upon the specifications needed for optical devices.
4 Optical Compensator of Black PhosphorusAn optical compensator, also referred to as a waveplate, is an optical device used in polarized light microscopy. Optical anisotropy is studied using a polarized light microscope with accessory plates that are divided into two primary categories: retardation plates, which have a fixed optical path difference, and compensators, which have variable optical path lengths [10]. Compensators and retarders come in two standard forms: half-wave plates (HWPs) and quarter-wave plates (QWPs), which require a birefringent material to accomplish the desired phase shift [11]. Traditional waveplates are composed of optically anisotropic quartz, mica, and gypsum minerals ground to a precise thickness and mounted between two optical windows having flat (plane) faces, which are designed to introduce a fixed amount of retardation between the orthogonal wavefronts passing through the crystal, recently manufacturers have also begun developing highly aligned and stretched linear organic polymers to produce anisotropic retardation plates [10].
A paper published by Mao et al in 2016 suggested that the optical properties of few-layer black phosphorus could be a viable candidate for future waveplate applications. The low-energy band gap of black phosphorus had in the past limited the study of its optical anisotropy to the near-infrared (NIR) spectrum, however Mao et al were successful in direct observation of the optical anisotropy of few-layer black phosphorus in the visible spectrum by using polarized optical microscopy [12]. The researchers tested the black phosphorus samples on a fused silica substrate and a 300 nm SiO2/Si substrate and found that the optical brightness of black phosphorus on the 300 nm SiO2/Si substrate changed dramatically with the rotation angle, which is demonstrated in other phosphorus allotropes from Section 2.2 of this review. The group's simulated and measured data can be found in Figure 6.
|
Fig.6 Simulated and measured anisotropic optical contrast and refractive indices for few-layer BP on 300 nm SiO2/Si and fused silica substrate Optical reflection and transmission schematic for multi-thinfilms system: (a) BP on 300 nm SiO2/Si and (b) fused silica. n stands for the refractive index of different media: air (n0), BP (n1), SiO2 (n2)and Si (n3). Angle-dependent optical contrast of (c) the 5 nm BP samples on 300 nm SiO2/Si and (d) fused silica under parallel polarizations. The wavelength of the incident light is 480 nm. Solid curves are fitted results using simulated equation. Insets are the corresponding optical images of the BP samples. The scale bar is 10 μm. (e) Optical contrast spectra along AC and ZZ crystalline direction of 5 nm thick BP on 300 nm SiO2/Si and fused silica substrates, respectively. (f) Measured refractive indices for 5 nm thick BP along AC and ZZ crystalline directions. The solid triangles and dots are the real and imaginary parts of the refractive indices, respectively. Reprinted with permission from reference [12]. Copyright 2016 by the American Chemical Society. |
In a time when renewable energy is becoming increasingly necessary to sustain our needs, one avenue that has gained a lot of attention is hydrogen generation from water and other sources. To be applicable, a photocatalyst with a suitable conduction band energy (around 2.0 eV to utilize solar energy effectively) is necessary for transferring the photogenerated electrons to water [13]. The added challenge of being stable in water makes it difficult to develop new visible-light-driven photocatalysts, especially simple elemental ones that are economically viable [13].
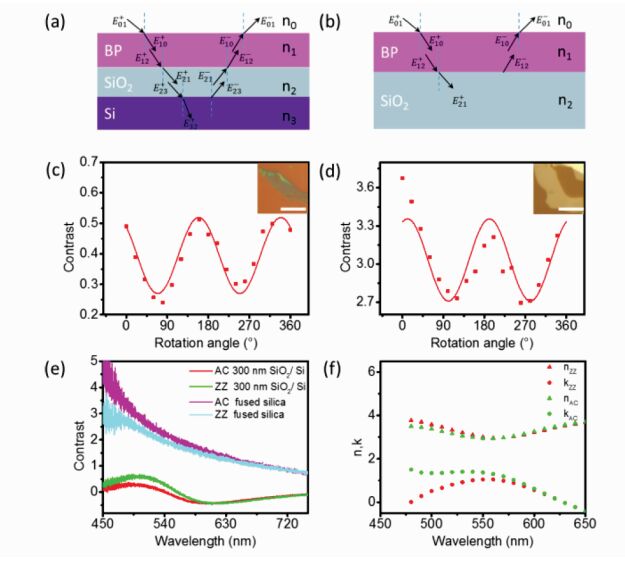
One work conducted by Lee et al [14], published in 2015, looked to create a hybrid photocatalyst using semiconducting 2D black phosphorus with titanium dioxide (TiO2). Black phosphorus layers (usually 1-3 phosphorene sheets), compared to graphene and MoS2 based alternatives, have higher carrier mobility and both p-type and n-type configurations in field-effect transistor (FET) sensors, however few-layered black phosphorus tends to be unstable due to oxygen and humidity, so a stabilizing agent is introduced in the form of TiO2. Figure 7 shows a model of the hybrid photocatalyst, along with additional data from the article. The researchers found that the hybrid photocatalyst held an approximately 92% efficiency after 15 runs, which is a vast improvement pure few layered black phosphorus, which dissolves in air after several uses [14].
|
Fig.7 (a) Modeled mono-, bi-layer, and tri-layered BP structures, (b) TiO2 substitution on BP structure, and (c) line profile (top panel) and its elemental mapping (bottom panel) of P, Ti, and O elements in BP@TiO2 hybrid photocatalyst. Reproduced with permission under Creative Commons License from reference [14] |
Other work being done with phosphorus photocatalysts includes a 2015 article by Shen et al. in which they produce a purely elemental heterostructure by ball milling black phosphorus with red phosphorus, creating high visible-light-driven (VLD) photocatalytic activity comparable to that of CdS [15]. The authors used 50%(mass fraction) black phosphorus in the mixture, and found that varying the black phosphorus amount, both higher and lower, resulted in poorer results. They reported 85% activity after 3 cycles [15], which is less than the TiO2 hybrid photocatalyst produced by Lee et al. [14], but appears to be a step in the right direction for the future of photocatalysts.
Red phosphorus can also be used as a photocatalyst. Wang et al. measured the rate of hydrogen gas production by irradiating 100 mL of solution (50 mg of products in 5 mL of methanol, as a hole sacrificial agent, and 95 mL of water) using a 300 watt Xe lamp, with H2 being produced at a rate of 0.08 μmol/h for crystalline red phosphorus. Amorphous red phosphorus was found to be only half as effective, possibly because the surface area of the crystalline red phosphorus (about 39 m2/g) is larger than that of amorphous red phosphorus (about 18 m2/g), i.e. increased surface area and a decrease in the number of electron-hole trapping centers [13]. To confirm the water photolysis process, a control experiment in the absence of methanol was performed. The results confirmed that H2 comes from photoreduction of water, and the rate of H2 formation also decreased by about 4 times in the absence of methanol, reaffirming that methanol most likely functions as a sacrificial agent to consume the photogenerated holes and improve the efficiency of red phosphorus [13]. It should also be noted that the activity was increased by approximately 12 times after loading 1%(mass fraction) Pt as a co-catalyst, created via the photo-reduction of H2PtCl6.
A 2016 paper by Hu et al. [16] shows that the rate of hydrogen gas formation using red phosphorus has greatly improved over time. The group tested two different approaches, the first being micro-fibrous red phosphorus grown on a silicon dioxide fiber and the second being "smashed-fibrous" red phosphorous, which was ultrasonically smashed from bulk. Using methanol as a sacrificial agent and Pt as a co-catalyst, like the Wang et al. paper [13], and under visible light irradiation, the team measured a hydrogen gas production rate of 196 μmol/(h·g). However, the paper justifies that since silicon dioxide is photoinactive and phosphorus comprises 31%(mass fraction) of the red phosphorus-SiO2 fiber, the true rate of hydrogen production is estimated to be around 633 μmol/(h·g). The group obtained a similar value of 684 μmol/(h·g) for the "smashed-fibrous" red phosphorus, with these rates being the highest values among purely elemental photocatalysts for visible light-driven hydrogen evolution from water [16]. A majority of the authors contributed to a 2017 review paper that performed an extensive overview of neutral P, phosphide (P3-), phosphate (P5+), and phosphorus composite/doped photocatalysts, including theoretical models [17].
A composite material that has been tested as a photocatalyst is P-CdS (red phosphorus and cadmium sulfide). The experimental conditions for measuring the rate of hydrogen gas production were quite similar to the Wang (2012) paper [13]: the team, Shi et al, irradiated a 100 mL solution (50 mg of photocatalysts in 100 mL of 0.1 mol·L-1 Na2S and 0.1 mol·L-1 Na2SO3 aqueous solution) using a 300 watt Xe lamp, which reduced Pt in added H2PtCl6 to create a 1%(mass fraction) Pt co-catalyst [18]. While pure CdS demonstrates a hydrogen generation of 364.5 μmol/(h·g), 10%(mole fraction) of red phosphorus added to the material (P0.1-CdS) multiplied this H2 generation by approximately 2.53, yielding a value of 923 μmol/(h·g) [18].
However, due to rising costs and availability of platinum metal, work has been done to try and replace it in the catalytic process. This is yet another avenue where red phosphorus has proved useful. Research done by Ye et al. discovered that while molybdenum phosphide (MoP) and molybdenum disulfide (MoS2) have proven to be effective independently at varying degrees, a composite material of the formula MoS2(1-x)Px (x ranging from 0 to 1) has shown great promise, with x = 0.53 demonstrating maximum performance of the material [19]. While this is not directly referencing red phosphorus as a photocatalyst on its own, it does demonstrate its versatility in generating hydrogen gas from water in the production of a non-precious metal replacement to platinum as a catalytic material through annealing with MoS2.
As a counter to the Lee (2015) article [14], rather than mixing TiO2 with 2D black phosphorus, work done by Ansari S A and Cho M H in 2016 introduced red phosphorus into TiO2, which caused a shift in the light absorption ability from UV to the visible spectrum, and confirmed that the optimal RP loading and milling time can effectively improve the visible light driven-photocatalytic activity of TiO2 [20]. The positives of their methods include the ease of creating the RP-TiO2-12 h nanohybrids, the cost effectiveness of using commercially available red phosphorus, and the use of utilizing TiO2, which is popular for its conversion of solar energy, hydrogen generation via water splitting reactions, and dye degradation [20].
6 Black Phosphorus as a Saturable Absorber and ModulatorA saturable absorber is an optical component with a certain optical loss, which is reduced at high optical intensities [21]. A Polish team led by Sotor J generated ultrashort pulses with Er-doped and Tm-doped fiber lasers of 272 fs centered at 1560.5 nm and 739 fs centered at 1910.5 nm, respectively. These laser fibers contained approximately 300 nm thick layers of black phosphorus, and transmission increased by about 4.4% [22]. Pulse speeds of 805 fs and 940 fs have also been reported [23, 24]. Jiang et al. [25] found similar results using black phosphorus as a saturable absorber, however they also used a thulium/holmium-doped fiber laser (THDFL). This particular laser demonstrated a 100 nm wavelength range could be achieved from 1832 nm to 1935 nm by adjusting the pump power. While this work was conducted in the near-IR region, work has also been done on the mid-IR region (between 2500 nm and 25000 nm). Qin et al. [26] worked with a 2.8 μm passively Q-switched Er:ZBLAN fiber laser using multi-layer black phosphorus as a saturable absorber. They found that the laser delivered a maximum average output power of 485 mW with a pulse energy of 7.7 μJ, pulse width of 1.18 μs, and repetition rate of 63 kHz. Many of the team members also worked on another project which investigated wavelengths of 1.03 μm, 1.93 μm, and 2.72 μm, which yielded similar results for multilayered black phosphorus, noting that saturable absorption at 2.72 μm was reaching the material's absorption limit [27]. Similarly, Li et al. [28] used a passively Q-switched singly Ho3+-doped fluoride fiber laser emitting at 2970.3 nm and mode-locked Ho3+/Pr3+ co-doped fluoride fiber laser centered at 2866.7 nm, both utilizing the same black phosphorus saturable absorber. Here, they generated a pulse duration of 8.6 ps at a repetition rate of 13.987 MHz for the holmium/praseodymium laser (output power and pulse energy were 87.8 mW and 6.28 nJ, respectively) and a pulse duration of 2.41 μs at a repetition rate of 62.5 kHz for the holmium laser (the output power and pulse energy were 308.7 mW and 4.93 μJ, respectively). Lastly, work done by Fan et al. [29] utilizing a passively Q-switched Er:Lu2O3 laser operation at 2.84 μm generated a minimum pulse duration of 359 ns with the highest average output power of 755 mW and a pulse energy of 7.1 μJ. These results are all significant because very few current saturable absorbers work stably in the mid-infrared spectral regime, and several different experiments confirm that black phosphorus is a cheap and viable candidate [26].
It is also worth noting that research has been done investigating the damage done to the black phosphorus saturable absorber.While investigating black phosphorus as a reliable material for a saturable absorber, Lee et al. [24] found that the material was capable of handling operations with a high intracavity power (greater than the power level which would damage BP in a blocking interaction) when involved in a non-blocking interaction scheme using a side-polished fiber for passively mode-locked pulsed lasers. They connected the black phosphorus-deposited side-polished fiber to the cavity set of their set-up at a high-powered laser setting of 23.3 dBm for 168 hours and found that the repetition rate (3.82 MHz) stayed constant throughout the exposure with only some fluctuation in spectral width (14.2 nm) centered at 1558.8 nm [24].
The major difference between a saturable absorber and an optical modulator has to do with the type of Q-switching involved. For passive Q-switching, which has been discussed, the losses are automatically modulated with a saturable absorber, where the pulse is formed as soon as the energy stored in the gain medium has reached a sufficiently high level [30]. However, for active Q-switching, the losses are modulated with an active control element (active Q-switch) typically either an acousto-optic or electro-optic modulator, where the pulse is formed shortly after an electrical trigger signal arrives. There are also mechanical Q switches such as spinning mirrors, used as end mirrors of laser resonators. In any case, the achieved pulse energy and pulse duration depends on the energy stored in the gain medium, i.e. on the pump power and the pulse repetition rate [30]. Research into using black phosphorus as a saturable absorber demonstrated that it could also be used as an optical modulator [25, 27, 29]. A review of 2D layered materials for use as optical modulators was already written in detail by Sun Z, Martinez A, and Wang F [31]. Of note, Sun et al. stated that black phosphorus was an attractive material for mid-and near-IR optoelectronics due to the fact that few-layer phosphorene remains direct for all sample thicknesses. However, the major setback to using black phosphorus in this application is its sensitivity to oxygen and humidity when in mono-or few-layers, requiring that in ambient conditions, the material be hermetically sealed [31].
7 Photoconductivity and Photodetection of Both Black and Red PhosphorusThe study of black phosphorus as an infrared photodetector isn't new, with notable research dating back to work done by Baba et al. [32] in 1989. Here, the team was able to determine that black phosphorus crystals demonstrated photoconductive properties with a linear response for light intensity and a selective response for polarized light in the near-IR region. Repeated work in 1991 showed that photoconduction was possible up to the mid-IR region as well [33]. However, while a paper published in 2001 showed that semiconducting black phosphorus could show metallic photoconduction at high pressure [34], it would still be another couple decades when 2D materials became a major research interest that this property would be reinvestigated. From a paper published in 2015 by Yuan et al. [35], they were able to produce photo-response rise times of linear dichroic black phosphorus photodetectors upwards of 40 μs, which is reported as a major improvement over previously reported photodetectors based on layered chalcogenides, which produce photo-response rise times on the order of milliseconds and slower. They were also able to show polarization sensitivity over a broad bandwidth from approximately 400 nm to 3750 nm (UV/Vis-to-mid IR region), affirming the near-IR results from the other work.
Since certain graphene chiralities lack a bandgap, graphene photodetectors can suffer from very high dark current, where as layered black phosphorous is ideal for photodetector applications due to its narrow but finite bandgap. A paper published by Youngblood et al. [36] showed that fabricated devices utilizing the bandgap of black phosphorus operating under bias with very low dark current attained an intrinsic responsivity up to 135 mA·W-1 and 657 mA·W-1 in 11.5-nm-thick and 100-nm-thick devices, respectively, at room temperature.
Multilayer black phosphorus has also been used as a multispectral photodetector for use in imaging. Research conducted by IBM scientists Engel M, Steiner M, and Avouris P demonstrated that a black phosphorus photodetector could not only handle imaging simple shapes, but also complex text patterns using scanning electron microscopy [37]. Their results are shown in Figure 8. This work demonstrated not only the application of black phosphorus photodetectors in the visible and near-IR spectral regime, but the clear imaging quality taken from the novel material [37].

|
Fig.8 (a) Scanning electron micrographs of test patterns on glass cover slides with feature sizes and pitch of 2000, 1000, and 500 nm. Scale bars are 10 μm; (b) Measured images excited at λIR = 1550 nm (top row) and simulated images (bottom row) Scale bars are 10, 5, and 2 μm. (c) Experimental image excited at λIR = 532 nm (top) exhibiting submicron features, along with the simulated image (bottom). Adapted with permission from reference [37]. Copyright 2014 by American Chemical Society. |
An article by Penillard et al. [38] published in 2016 sought to use the photoconductive properties of black phosphorus to create optoelectronic devices, such as a microwave photoconductive switch, which allows optical control over the magnitude and phase of microwave signals. The authors note that 2D black phosphorus is a very promising material for optoelectronic applications, because it has a direct band gap of 0.3 eV in bulk configuration, which is converted to a bandgap of 2 eV when reduced to an atomically thin layer [38].
The paper published in 2012 by Wang et al. [13] discussed previously also demonstrated the photoconductive properties of red phosphorus. While the paper focused on the applications of these properties, the researchers went into detail about which properties of red phosphorus come from photoconductivity.The group put 100 mg of red phosphorus inside a quartz tube, sealed under vacuum, and heated to 650 ℃ to create a roughly 10-μm-thick phosphorus thin film. The photocurrent was 0.09 A/cm2 when light was applied and drops to 0.002 A/cm2 without illumination, which verifies that electrons and holes are generated over red phosphorus under illumination [13]. A mobility of 8.05 cm2/V·s was obtained, indicating that red phosphorus should be considered a p-type semiconductor.
8 PerspectiveExtensive studies have been carried on the development of inexpensive, versatile materials. Phosphorus has gradually attracted researchers' attention, with black phosphorus and red phosphorus being the two main allotropes of interest. The last few years have seen a dramatic increase in the study of phosphorus and its optical properties.
The nonlinear optical properties of black phosphorus flakes are anisotropic and can be tuned by the black phosphorus film thickness, which is not seen in common 2D layered materials used today. Phosphorus nanotubes utilize a possibly new allotrope of phosphorus, blue phosphorus, and exhibit optical reflectance dependent upon the angle of incoming polarized light. Phosphorus nanotubes also seem to exhibit these reflective properties differently depending upon which allotrope is used for the tube frame, black phosphorus or blue phosphorus. Since phosphorus displays a wide range of optical reflections depending upon allotrope, it makes it a desirable material for use in a variety of applications. Phosphorus also satisfies the needs of a visible light photoconductor, with a tunable band gap in the range of 0.3 eV to 2 eV. With its semiconducting properties, it makes phosphorus applicable in areas where graphene (which exhibits metallic conduction characteristics) would be unusable.
The optical properties of phosphorus give rise to many theoretical applications, but there are a few where phosphorus allotropes are shown to work. Whereas traditional waveplates are composed of optically anisotropic quartz, mica, and gypsum minerals, studies have shown that few-layer black phosphorus is a viable candidate for future waveplates due to its refractive index. With green technology being the direction our society is heading towards, phosphorus has proven an effective photocatalyst in H2 generation from water, both in the black and the crystalline red allotropes. Black phosphorus layers have even been shown to exhibit n-type and p-type semiconducting properties in FET sensors. Mixtures of black phosphorus with TiO2, red phosphorus with TiO2, and even black phosphorus with red phosphorus have also proven effective as visible-light-driven photocatalysts.
Future work will need to address a few key issues, such as improving efficiency over dozens of runs, finding lasting solutions to the air and humidity sensitivity of phosphorene, and being effective enough to replace current heavy metals in devices, which can be toxic. On the topic of toxicity, working with phosphorus creates inherent risk, as white phosphorus production is a real and dangerous issue in the field. Caution must always be taken when working with phosphorus allotropes. However, even with the setbacks and risks, phosphorus is proving to be the latest in a series of wonder materials as technology shifts to the nanoscale. It is also worth noting that while a lot of research has been done on black phosphorus nanomaterials, there are far fewer papers on the properties of red phosphorus. From the works that were covered in this review, it seems that red phosphorus could be the next material investigated for its optical properties.
| [1] | Corbridge D E C. Phosphorus:An Outline of Its Chemi-stry, Biochemistry, and Technology[M]. Amsterdam: Elsevier, 1990. |
| [2] | Bachhuber F, von Appen J, Dronskowski R, Schmidt P, Nilges T, Pfitzner A, Weihrich R. The extended stability range of phosphorus allotropes[J]. Angewandte Chemie International Edition, 2014, 53(43): 11629–11633. DOI:10.1002/anie.201404147 |
| [3] | Ruck M, Hoppe D, Wahl B, Simon P, Wang Y, Seifert G. Fibrous red phosphorus[J]. Angewandte Chemie International Edition, 2005, 44(46): 7616–7619. DOI:10.1002/(ISSN)1521-3773 |
| [4] | Smith J B, Hagaman D, Ji H F. Growth of 2D black phosphorus film from chemical vapor deposition[J]. Nanotech-nology, 2016, 27: 215602. DOI:10.1088/0957-4484/27/21/215602 |
| [5] | Li D, Jussila H, Karvonen L, Ye G, Lipsanen H, Chen X, Sun Z. Polarization and thickness dependent absorption properties of black phosphorus:new saturable absorber for ultrafast pulse generation[J]. Scientific Reports, 2015, 5: 15899. DOI:10.1038/srep15899 |
| [6] | Hu T, Hashmi A, Hong J. Geometry, electronic structures and optical properties of phosphorus nanotubes[J]. Nanotechnology, 2015, 26(41): 415702. DOI:10.1088/0957-4484/26/41/415702 |
| [7] | Zhu Z, Tománek D. Semiconducting layered blue phospho-rus:a computational study[J]. Physical Review Letters, 2014, 112: 176802. DOI:10.1103/PhysRevLett.112.176802 |
| [8] | Asahina H, Morita A. Band structure and optical properties of black phosphorus[J]. Journal of Physics C:Solid State Physics, 1984, 17(11): 1839–1852. DOI:10.1088/0022-3719/17/11/006 |
| [9] | Mohr P J, Taylor B N, Newell D B. The 2010 CODATA recommended values of the fundamental physical constants[J]. Reviews of Modern Physics, 2012, 84: 1527. DOI:10.1103/RevModPhys.84.1527 |
| [10] | Collins R W. Automatic rotating element ellipsometers:calibration, operation, and real-time applications[J]. Review of Scientific Instruments, 1990, 61(8): 2029–2062. DOI:10.1063/1.1141417 |
| [11] | Peters T, Ivanov S S, Englisch D, Rangelov A A, Vitanov N V, Halfmann T. Variable ultra-broadband and narrowband composite polarization retarders[J]. Applied Optics, 2012, 51(31): 7466–7474. DOI:10.1364/AO.51.007466 |
| [12] | Mao N, Tang J, Xie L, Wu J, Han B, Lin J, Deng S, Ji W, Xu H, Liu K, Tong L, Zhang J. Optical anisotropy of black phosphorus in the visible regime[J]. Journal of the American Chemical Society, 2016, 138(1): 300–305. DOI:10.1021/jacs.5b10685 |
| [13] | Wang F, Ng W K H, Yu J C, Zhu H, Li C, Zhang L, Liu Z, Li Q. Red phosphorus:an elemental photocatalyst for hydrogen formation from water[J]. Applied Catalysis B, 2012, 111-112: 409–414. DOI:10.1016/j.apcatb.2011.10.028 |
| [14] | Lee H U, Lee S C, Won J, Son B C, Choi S, Kim Y, Park S Y, Kim H S, Lee Y C, Lee J. Stable semiconductor black phosphorus (BP)@Titanium dioxide (TiO2) hybrid photocatalysts[J]. Scientific Reports, 2015, 5: 8691. DOI:10.1038/srep08691 |
| [15] | Shen Z, Sun S, Wang W, Liu J, Liu Z, Yu J C. A black-red phosphorus heterostructure for efficient visible-light-driven photocatalysis[J]. Journal of Materials Chemistry A, 2015, 3: 3285–3288. DOI:10.1039/C4TA06871H |
| [16] | Hu Z, Yuan L, Liu Z, Shen Z, Yu J C. An elemental phosphorus photocatalyst with a record high hydrogen evolution efficiency[J]. Angewandte Chemie International Edition, 2016, 55: 9580–9585. DOI:10.1002/anie.201603331 |
| [17] | Hu Z, Shen Z, Yu J C. Phosphorus containing materials for photocatalytic hydrogen evolution[J]. Green Chemistry, 2017, 19: 588–613. DOI:10.1039/C6GC02825J |
| [18] | Shi Z, Dong X, Dang H. Facile fabrication of novel red phosphorus-CdS composite photocatalysts for H2 evolution under visible light irradiation[J]. International Journal of Hydrogen Energy, 2016, 41: 5908–5915. DOI:10.1016/j.ijhydene.2016.02.146 |
| [19] | Ye R, del Angel-Vicente P, Liu Y, Arellano-Jimenez M J, Peng Z, Wang T, Li Y, Yakobson B I, Wei S H, Yacaman M J, Tour J M. High-performance hydrogen evolution from MoS2(1-x)Px solid solution[J]. Advanced Materials, 2016, 28: 1427–1432. DOI:10.1002/adma.v28.7 |
| [20] | Ansari S A, Cho M H. Highly visible light responsive, narrow band gap TiO2 nanoparticles modified by elemental red phosphorus for photocatalysis and Photoelectrochemical applications[J]. Scientific Reports, 2016, 6: 25405. DOI:10.1038/srep25405 |
| [21] | Garside B K, Lim T K. Laser mode locking using saturable absorbers[J]. Journal of Applied Physics, 1973, 44(5): 2335–2342. DOI:10.1063/1.1662561 |
| [22] | Sotor J, Sobon G, Macherzynski W, Paletko P, Abramski K M. Black phosphorus-a new saturable absorber material for ultrashort pulse generation[J]. Applied Physics Letters, 2015, 107: 051108. DOI:10.1063/1.4927673 |
| [23] | Luo Z C, Liu M, Guo Z N, Jiang X F, Luo A P, Zhao C J, Yu X F, Xu W C, Zhang H. Microfiber-based few-layer black phosphorus saturable absorber for ultra-fast fiber laser[J]. Optics Express, 2015, 23(15): 20030–20039. DOI:10.1364/OE.23.020030 |
| [24] | Lee D, Park K, Debnath P C, Kim I, Song Y W. Thermal damage suppression of a black phosphorus saturable absorber for high-power operation of pulsed fiber lasers[J]. Nanotechnology, 2016, 27: 365203. DOI:10.1088/0957-4484/27/36/365203 |
| [25] | Jiang T, Yin K, Zheng X, Yu H, Cheng X A. Black phosphorus as a new broadband saturable absorber for infrared passively Q-switched fiber lasers[J]. 2015, arXiv: 1504. 07341[physics. optics]. arXiv. org e-Print archive. |
| [26] | Qin Z, Xie G, Zhang H, Zhao C, Yuan P, Wen S, Qian L. Black phosphorus as a saturable absorber for the Q-switched Er:ZBLAN fiber laser at 2.8μm[J]. Optics Express, 2016, 23(19): 24713–24718. |
| [27] | Kong L, Qin Z, Xie G, Guo Z, Zhang H, Yuan P, Qian L. Multilayer black phosphorus as broadband saturable absorber for pulsed lasers from 1 to 2.7μm wavelength[J]. Laser Physics Letters, 2016, 13: 045801. DOI:10.1088/1612-2011/13/4/045801 |
| [28] | Li J, Luo H, Zhai B, Lu R, Guo Z, Zhang H, Liu Y. Black phosphorus:a two-dimension saturable absorption material for mid-infrared Q-switched and mode-locked fiber lasers[J]. Scientific Reports, 2016, 6: 30361. DOI:10.1038/srep30361 |
| [29] | Fan M, Li T, Zhao S, Li G, Gao X, Yang K, Li D, Kränkel C. Multilayer black phosphorus as saturable absorber for an Er:Lu2O3 laser at~3μm[J]. Photonics Research, 2016, 4(5): 181–186. DOI:10.1364/PRJ.4.000181 |
| [30] | Degnan J J. Optimization of passively Q-switched lasers[J]. IEEE Journal of Quantum Electron, 1995, 31(11): 1890–1901. DOI:10.1109/3.469267 |
| [31] | Sun Z, Martinez A, Wang F. Optical modulators with 2D layered materials[J]. Nature Photonics, 2016, 10: 227–238. DOI:10.1038/nphoton.2016.15 |
| [32] | Baba M, Takeda Y, Shibata K, Ikeda T, Morita A. Optical properties of black phosphorus and its application to the infrared detector[J]. Japanese Journal of Applied Phy-sics, 1989, 28(11): 2104–2106. |
| [33] | Baba M, Nakamura Y, Shibata K, Morita A. Photoconduction of black phosphorus in the infrared region[J]. Japanese Journal of Applied Physics, 1991, 30(7A): 1178–1181. |
| [34] | Akahama Y, Kawamura H. Optical and electrical studies on band-overlapped metallization of the narrow-gap semiconductor black phosphorus with layered structure[J]. Physica Status Solidi(b), 2001, 223: 349–353. DOI:10.1002/1521-3951(200101)223:1<349::AID-PSSB349>3.0.CO;2-F |
| [35] | Yuan H, Liu X, Afshinmanesh F, Li W, Xu G, Sun J, Lian B, Curto A G, Ye G, Hikita Y, Shen Z, Zhang S C, Chen X, Brongersma M, Hwang H Y, Cui Y. Broadband linear-dichroic photodetector in a black phosphorus vertical p-n junction[J]. Nature Nanotechnology, 2015, 10: 707–713. DOI:10.1038/nnano.2015.112 |
| [36] | Youngblood N, Chen C, Koester S J, Li M. Waveguide-integrated black phosphorus photodetector with high responsivity and low dark current[J]. Nature Photonics, 2015, 9: 247–252. |
| [37] | Engel M, Steiner M, Avouris P. Black phosphorus photodetector for multispectral, high-resolution imaging[J]. Nano Letters, 2014, 14(11): 6414–6417. DOI:10.1021/nl502928y |
| [38] | Penillard A, Tripon-Canseliet C, Rosticher M, Maksimo-vic I, Liu Z, Géron E. Effective photoconductivity of exfoliated black phosphorus for optoelectronic switching under 1.55μm optical excitation[J]. Journal of Applied Phy-sics, 2016, 119: 024506. DOI:10.1063/1.4939615 |




