Triphenylphosphine (PPh3), an important organic substance with a lone pair of electrons on phosphine, is a strong Lewis base and easy to form complexes with transition metals. PPh3 is ubiquitous for its nucleophilicity and reducing character. It is widely used in the synthesis of organic and organometallic compounds, such as Wittig reaction, Mitsunobu reaction, Mukaiyama-Corey lactonization, Appel reaction, Staudinger reaction, and as ligand in carbon-carbon bond formation reactions (e.g., Suzuki, Heck, and Negishi coupling)[1]. In the fields of fluorescent probes, PPh3 was often used for direct imaging of azide-bearing molecules based on bioorthogonal Staudinger ligation in living cells[2-4], which could form an stable amide bond in complex biological environment, such as in living cells and animals[5]. This reaction has been employed in a wide range of applications, including modification of cell surfaces[6], protein engineering[7], specific labeling of nucleic acids[8], proteomic studies, and as a general tool for bioconjugation[9,10]. Very recently, several PPh3-containing fluorescent probes for the discrimination of GSNO or HNO have been reported[11-16].
The extensive use of PPh3 is inevitable to cause phosphorous pollution to the environment and would pose a potential danger to human health. Traditionally, analysis of PPh3 in various matrices has been accomplished by spectrophotometric[17], titrimetric[18], chromatographic[19], and high performance liquid chromatography (HPLC)[20] methods. Unfortunately, those methods suffer limitations on the sensitivity and ease of use. A convenient and effective detection method for PPh3 is therefore desirable. Fluorescent techniques are extremely attractive due to their simplicity, high sensitivity and real-time detection[21]. However, little attention had been paid for the detecting of PPh3 in the research area of fluorescent probe. As a part of our continued effort for development of fluorescent probes for neutral organic substance[22-25], herein, we report the first fluorescent probe for PPh3 mimicking Staudinger ligation with a different arrangement of functionalities. As showed in Scheme 1, our design concept was to mask a hydroxyl-containing fluorophore as 2-azidophenylacetic ester. Such a latent fluorophore may react with PPh3 to undergo a rapid intramolecular acyl transfer through aza-ylide intermediate to release the fluorophore after hydrolysis[26].
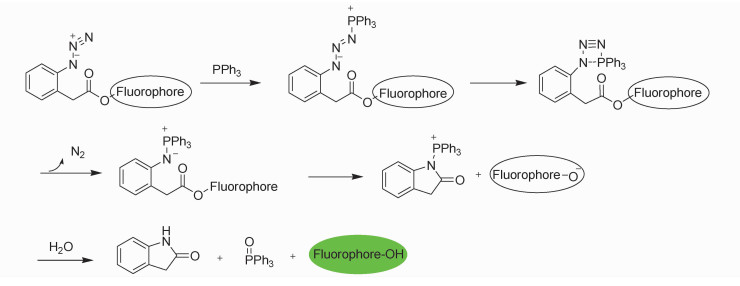
|
Scheme1 The design concept and the plausible sensing mechanism |
All reagents were purchased from commercial suppliers and used without further purification. Solvents used were purified by standard methods prior to use. Acetonitrile in chromatographic purity and deionized water were used in detection. 1HNMR spectra were recorded on a VARIAN Mercury 400 MHz spectrometer. 1HNMR chemical shifts (δ) are given in ppm (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet) downfield from Me4Si, determined by chloroform (δ = 7.26 ppm) and dimethyl sulfoxide (δ = 2.5 ppm). 13CNMR spectra were recorded on a VARIAN Mercury 100 MHz spectrometer. 13CNMR chemical shifts (δ) are reported in ppm with the internal CDCl3 and d6-DMSO at 77.0 and 39.4 as standard, respectively. Mass spectrometric measurements were performed by AB SCIEX Triple TOFTM 5600+ mass spectrometry. Fluorescence spectra were recorded on FluoroSENS spectrophotometer. UV/Vis spectra were recorded on Perkin-Elmer Lambda 35 UV/Vis spectrophotometer at room temperature. All spectra were recorded at room temperature except for the fluorescence microscopic images.
1.2 Synthesis2-(2-Azidophenyl) acetic acid was prepared according to the reported method[27].
Probe 1: Under N2, a solution of 2-(2-azidophenyl) acetic acid (106 mg, 0.6 mmol), DCC (124 mg, 0.6 mmol), and DMAP (22 mg, 0.2 mmol) in 10 mL of CH2Cl2 were added dropwise in a solution of fluorescein (66 mg, 0.2 mmol) in 3 mL of CH2Cl2 at ambient temperature. The reaction mixture was stirred for 3 h. Solvent was evaporated in vacuo and the residue was put onto preparative silica gel plates (developed with CH2Cl2) to isolate probe 1 as a white solid after evaporation of solvent (75 mg, 83%).
1HNMR (400 MHz, CDCl3): 8.03 (d, 1H, J = 8.0 Hz), 7.65 (m, 2H), 7.38 (td, 2H, J = 8.0, 2.0 Hz), 7.31 (dd, 2H, J = 8.0 Hz, 1.0 Hz), 7.21 (d, 2H, J = 8.0 Hz), 7.15 (m, 3H), 7.09 (s, 2H), 6.82 (d, 4H, J = 4.0), 3.85 (s, 4H). 13CNMR (100 MHz, CDCl3): 169.20, 169.02, 153.01, 152, 16, 151.55, 138.83, 135.35, 131.56, 130.10, 129.21, 128.98, 126.06, 125.27, 125.03, 124.79, 124.05, 118.32, 117.69, 116.51, 110.34, 81.65, 36.84. HRMS-ESI: [M]+ calculated for C36H22N6O7Na+1: 673.1442; found: 673.1438.
2 Results and DiscussionThe implementation of our design concept was exemplified by the fluorescein-based probe 1 as showed in scheme 2. Fluorescein is a well-known highly fluorescent dye and is non-fluorescent when the two hydroxyl groups were both acylated. In this study, fluorescein reacted with excess of 2-azidophenylacetic acid in the presence of DCC and DMAP to generate the desired masked fluorophore. Probe 1 may react with PPh3 in a sequential manner to release highly fluorescent mono-deprotected fluorescein and fluorescein. It is known that even mono-deprotected species of probe 1 is also strong fluorescent with almost identical emission maxima[28].
Probe 1 does not have obvious absorption band around 490 nm and is almost non-fluorescent. When probe 1 (1 mol/L) was subjected to PPh3 (10 mol/L, 10 equiv) in DMSO/PBS buffer (0.5:100, V/V, 10 mmol/L, pH 7.4) at 25 ℃, there were distinct changes in the absorption and emission of probe 1 (Figure 1). Upon addition of PPh3, the absorption at 490 nm increased with increasing time, fluorescence intensity at 515 nm enhanced concurrently and reached the maximum after about 60 min. These preliminary results indicated that the expected chemical reaction between the PPh3 and probe 1 released fluorescein.
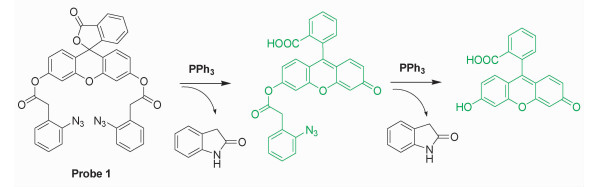
|
Scheme2 Structure of probe 1 and the proposed sensing reactions |

|
Fig.1 Time-dependent absorption and fluorescence emission (λex = 470 nm) profiles of probe 1 (1 μmol/L) in DMSO/PBS buffer (0.5:100, V/V, 10 mmol/L, pH 7.4) with PPh3 (10 μmol/L) after certain periods of time (0, 1, 4, 7, 10, 13, 16, 19, 22, 25, 28, 31, 34, 37, 40, 43, 46, 49, 52, 57, 70, and 80 min) |
With the positive results obtained, we then carried out concentration-dependent fluorescence response studies toward various concentrations of PPh3. Upon incremental addition of PPh3 with concentration range of 0-20 μmol/L to DMSO/PBS buffer (0.5:100, V/V, 10 mmol/L, pH7.4) solution of probe 1 (1 μmol/L) at 25 ℃ and allowed to react for 60 min, a concurrently increased fluorescence at 515 nm was observed (Figure 2a). Further studies suggested a concentration-dependent response with linear character of probe 1 toward PPh3 ranged from 1×10-7 mol/L to 8×10-7 mol/L. The detection limit for PPh3 was estimated to be 10 nmol/L based on the signal-to-noise ratio (S/N=3), which suggested that our probe was fairly sensitive to detect PPh3.
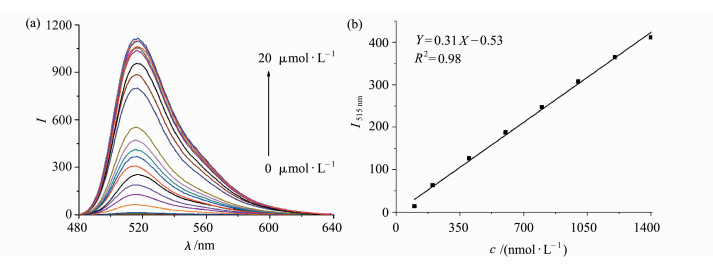
|
Fig.2 (a) Fluorescence spectra (λex = 470 nm) of probe 1 (1 μmol/L) upon addition of increasing concentrations of PPh3 (0-20 equiv.) in DMSO/PBS buffer (0.5:100, V/V, 10 mmol/L, pH 7.4) for 60 min; (b) concentration-dependent linear response of fluorescence intensities of the probe 1 (1 μmol/L) toward PPh3 monitored at 515 nm from 1×10-7 mol/L to 1.4×10-6 mol/L |
To investigate the selectivity, various potential interfering species were tested. Probe 1 was initially investigated in the presence of various metallic ions in DMSO/PBS buffer (0.5:100, V/V, 10 mmol/L, pH 7.4) at 25 ℃ by adding PPh3 (10 μmol/L) or 100 μmol/L of metallic ion to the solution of probe 1 (1 μmol/L) and incubated for 60 min prior to follow the fluorescence at 515 nm (Figure 3a). None of the metallic ion used caused any noticeable fluorescence enhancement, only PPh3 induced significant fluorescence (Figure 3a). Further investigations on competitive experiments wherein a mixture of PPh3 (10 μmol/L) and individual metallic ion (100 μmol/L) were incubated in DMSO/PBS buffer (0.5:100, V/V, 10 mmol/L, pH 7.4) at 25 ℃ for 60 min suggested that only PPh3 turned on obvious fluorescence (Figure 3b).
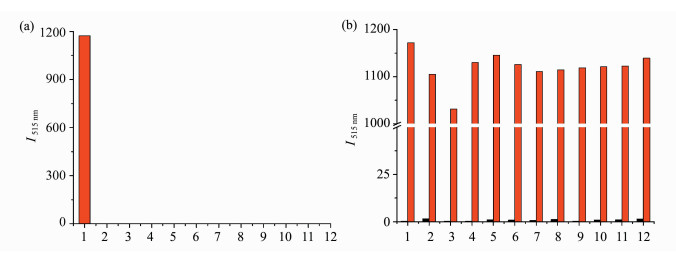
|
Fig.3 (a) The selectivity of probe 1 (1 μmol/L) toward PPh3 (10 μmol/L) and other substances (100 μmol/L) in DMSO/PBS buffer (0.5:100, V/V, 10 mmol/L, pH 7.4) for 60 min; (b) the selectivity of probe 1 (1 μmol/L) toward PPh3 (10 μmol/L) and metallic ions (100 μmol/L each) in DMSO/PBS buffer (0.5:100, V/V, 10 mmol/L, pH 7.4) for 60 min
(λex = 470 nm, λem = 515 nm) Black bar: the fluorescence intensity of only a single analyte with probe 1 Red bar: the fluorescence intensity of a mixture of analyte and PPh3 with probe 1 (1) PPh3, (2) Pd(OAc)2, (3) CuCl2·2H2O, (4) AlCl3·6H2O, (5) FeCl3, (6) FeSO4, (7) ZnCl2, (8) MnCl2, (9) CoCl2·6H2O, (10) BiCl3, (11) NiCl2·6H2O, (12) Cd(NO3)2·2H2O |
We subsequently tested more potential interfe-ring analytes of biothiols, nucleophiles, and various anions and the results are shown in Figure 4. Both incubation of probe 1 with individual analyte and competitive experiments with mixture of PPh3 and the applied interfering analyte provided evidence that only PPh3 elicited obvious fluorescence turn-on (Figure 4). Notably, although aryl azide functiona-lity is known to be reduced by NaSH or Na2S to produce aniline, we did not observe obvious fluorescence increment presumably due to the low nucleophilicity of aniline compared with aza-ylide species. Thus, we can conclude that probe 1 displayed excellent selectivity toward PPh3, namely, only strong fluorescence was visualized when probe 1 exposed to PPh3, which was not disturbed by other substances.
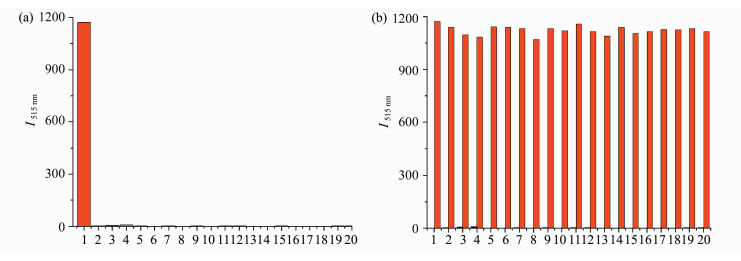
|
Fig.4 (a) The selectivity of probe 1 (1 μmol/L) toward PPh3 (10 μmol/L) and other substances (100 μmol/L) in DMSO/PBS buffer (0.5:100, V/V, 10 mmol/L, pH 7.4) for 60 min. Fluorescence intensity of a mixture of analyte and PPh3 with probe 1; (b) the selectivity of probe 1 (1 μmol/L) toward PPh3 (10 μmol/L) and other analytes (100 μmol/L each) in DMSO/PBS buffer (0.5:100, V/V, 10 mmol/L, pH 7.4) for 60 min (λex = 470 nm and λem = 515 nm) Black bar: the fluorescence intensity of only a single analyte with probe 1. Red bar: the fluorescence intensity of a mixture of analyte and PPh3 with probe 1 (1) PPh3, (2) Hcy, (3) GSH, (4) Cys, (5) NaN3, (6) NaClO, (7) H2O2, (8) NaHS, (9) NaHSO3, (10) Na2S2O3, (11) Na2S2O4, (12) Na2S2O5, (13) Na2SO4, (14) KClO4, (15) NaNO2, (16) NaOAc, (17) Mg(NO3)2, (18) Na2C2O4, (19) Na2S, (20) NaHSO3 |
The effect of pH on the fluorescence intensity and reactivity of probe 1 (1 μmol/L) was examined in the absence and presence of PPh3 in DMSO/PBS buffer (0.5:100, V/V, 10 mmol/L) at 25 ℃ for 60 min (Figure 5). Probe 1 is stable in pH range of 0.0-10.0. However, at high pH greater than 10.0, the ester bond of probe 1 started to be cleaved. In the presence of PPh3, the increased reaction rate at high pH environment was observed and the preferred pH range was 6.0-10.0.
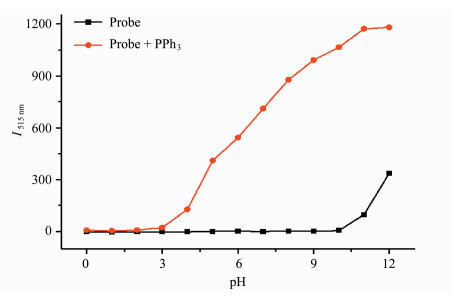
|
Fig.5 Effect of the pH value of solvent on the fluorescence intensity of probe 1 (1 μmol/L) with PPh3 (10 μmol/L) in DMSO/PBS buffer (0.5:100, V/V, 10 mmol/L, pH 7.4) for 60 min |
The above investigations revealed that probe 1 was superior to detect PPh3 with excellent sensitivity and selectivity. The big off-to-on (φ: 0→1) contrast ratio and dramatic change of fluorescence upon addition of PPh3 allow sensitive identification of PPh3 even with "naked eye" as shown in Figure 6. When probe 1 (1 μmol/L) was treated with PPh3 (10 μmol/L) in comparison with various metallic ions, anions, amino acids, reductants or oxidants (100 μmol/L each) in water containing 0.5% DMSO solution, only PPh3 resulted strong green fluorescence without any interference from other analyte.

|
Fig.6 Photographs of probe 1 (1 μmol/L) with PPh3 (10 μmol/L), reducing ions, natural amino acids and other metallic ions (100 μmol/L) in water containing 0.5% DMSO solution for 60 min at 25 ℃ taken under ambient light and under a hand-held UV lamp with λex = 365 nm (1) PPh3, (2) Hcy, (3) GSH, (4) Cys, (5) NaN3, (6) NaClO, (7) H2O2, (8) NaHS, (9) NaHSO3, (10) Na2S2O3, (11) Na2S2O4, (12) Na2S2O5, (13) Na2SO4, (14) KClO4, (15) NaNO2, (16) NaOAc, (17) Mg(NO3)2, (18) Na2C2O4, (19) Na2S, (20) NaHSO3, (21) Pd(OAc)2, (22) CuCl2·2H2O, (23) AlCl3·6H2O, (24) FeCl3, (25) FeSO4, (26) ZnCl2, (27) MnCl2, (28) CoCl2·6H2O, (29) BiCl3, (30) NiCl2·6H2O, (31) Cd(NO3)2·2H2O |
In summary, we have developed a novel fluorescent probe 1 with high selectivity and sensitivity for PPh3 by attaching 2-azidophenylacetyl functionality to fluorescein mimicking Staudinger ligation. Probe 1 is promising for the sensitive detection of PPh3, and the detection limit is about 1×10-8 mol/L measured in DMSO/PBS buffer. The sensitive detection of PPh3 can be viewed with "naked eye" in aqueous solution containing 0.5% DMSO.
Acknowledgements This research was supported by NSFC(21372063).| [1] | Cobb J E, Cribbs C M, Henke B R, Uehling D E, Hernan A G, Martin C, Rayner C M. e-EROS Encyclopedia of Reagents for Organic Synthesis[M]. New Jersey: John Wiley and Sons, 2005: 1-12. |
| [2] | Lemieux G A, de Graffenried C L, Bertozzi C R. A fluorogenic dye activated by the Staudinger ligation[J]. Journal of the American Chemical Society, 2003, 125(16): 4708–4709. DOI:10.1021/ja029013y |
| [3] | Chen X Q, Wang F, Hyun J Y, Wei T W, Qiang J, Ren X T, Shin I, Yoon J. Recent progress in the development of fluorescent, luminescent and colorimetric probes for detection of reactive oxygen and nitrogen species[J]. Chemical Society Reviews, 2016, 45(10): 2976–3016. DOI:10.1039/C6CS00192K |
| [4] | Hangauer M J, Bertozzi C R. A FRET-based fluorogenic phosphine for live-cell imaging with the staudinger ligation[J]. Angewandte Chemie International Edition, 2008, 120(13): 2428–2431. |
| [5] | Kohn M J, Breinbauer C R. The Staudinger ligation:a gift to chemical biology[J]. Angewandte Chemie International Edition, 2004, 43(24): 3106–3116. DOI:10.1002/(ISSN)1521-3773 |
| [6] | Saxon E, Luchansky S J, Hang H C, Yu C, Lee S C, Bertozzi C R. Investigating cellular metabolism of synthetic azidosugars with the Staudinger ligation[J]. Journal of the American Chemical Society, 2002, 124(50): 14893–14902. DOI:10.1021/ja027748x |
| [7] | Nilsson B L, Hondal R J, Soellner M B, Raines R T. Protein assembly by orthogonal chemical ligation methods[J]. Journal of the American Chemical Society, 2003, 125(18): 5268–5269. DOI:10.1021/ja029752e |
| [8] | Charles C, Wang Y, Seo T S, Li Z M, Ruparel H, Ju J Y. Site-specific fluorescent labeling of DNA using Staudinger ligation[J]. Bioconjugate Chemistry, 2003, 14(3): 697–701. DOI:10.1021/bc0256392 |
| [9] | Soellner M B, Dickson K A, Nilsson B L, Raines R T. Site-specific protein immobilization by Staudinger ligation[J]. Journal of the American Chemical Society, 2003, 125(39): 11790–11791. DOI:10.1021/ja036712h |
| [10] | Kohn M, Wacker R, Peters C, Schroder H, Soulere L, Breinbauer R, Niemeyer C M, Waldmann H. Staudinger ligation:a new immobilization strategy for the preparation of small-molecule arrays[J]. Angewandte Chemie International Edition, 2003, 42(47): 5830–5834. DOI:10.1002/(ISSN)1521-3773 |
| [11] | Liu C Y, Wu H F, Wang Z K, Shao C X, Zhu B C, Zhang X L. A fast-response, highly sensitive and selective fluorescent probe for the ratiometric imaging of nitroxyl in living cells[J]. Chemical Communications, 2014, 50(45): 6013–6016. DOI:10.1039/c4cc00980k |
| [12] | Liu P, Jing X T, Yu F B, Lv C J, Chen L X. A near-infrared fluorescent probe for the selective detection of HNO in living cells and in vivo[J]. Analyst, 2015, 140(13): 4576–4583. DOI:10.1039/C5AN00759C |
| [13] | Zhang D, Chen W, Miao Z R, Ye Y, Zhao Y F, King S B, Xian M. A reductive ligation based fluorescent probe for S-nitrosothiols[J]. Chemical Communications, 2014, 50(37): 4806–4809. DOI:10.1039/C4CC01288G |
| [14] | Jing X T, Yu F B, Chen L X. Visualization of nitroxyl (HNO) in vivo via a lysosome-targetable near-infrared fluorescent probe[J]. Chemical Communications, 2014, 50(91): 14253–14256. DOI:10.1039/C4CC07561G |
| [15] | Gong X Y, Yang X F, Zhong Y G, Chen Y H, Li Z. A mitochondria-targetable near-infrared fluorescent probe for imaging nitroxyl (HNO) in living cells[J]. Dyes Pigments, 2016, 131: 24–32. DOI:10.1016/j.dyepig.2016.03.046 |
| [16] | Sunwoo K, Bobba K N, Lim J Y, Park T, Podder A, Heo J S, Lee S G, Bhuniya S, Kim J S. A bioorthogonal "turn-on" fluorescent probe for tracking mitochondrial nitroxyl formation[J]. Chemical Communications, 2017, 53(10): 1723–1726. DOI:10.1039/C6CC09082F |
| [17] | McDonald D B, Shulman J. Spectrophotometric determination of triphenylphosphine in dilute solutions[J]. Analytical Chemistry, 1975, 47(8): 2023–2024. |
| [18] | Rao V R S, Aravamudan G. Oxidimetric determination of triphenylphosphine[J]. Talanta, 1969, 16(12): 1594–1596. DOI:10.1016/0039-9140(69)80223-7 |
| [19] | Carnes W J, Dean J A. Flame spectrophotometric study of yttrium[J]. Analytical Chemistry, 1961, 33(13): 1961–1962. DOI:10.1021/ac50154a059 |
| [20] | Haky J E, Baird D M, Falzone S. Revrsed phase high performance liquid chromatographic analysis of triphenylphosphine in a reaction mixture[J]. Analytical Letters, 1989, 22(11): 2637–2651. |
| [21] | Jung H S, Chen X Q, Kim J S, Yoon J. Recent progress in luminescent and colorimetric chemosensors for detection of thiols[J]. Chemical Society Reviews, 2013, 42(14): 6019–6031. DOI:10.1039/c3cs60024f |
| [22] | Zhang J, Bao X L, Zhou J L, Peng F F, Ren H, Dong X C, Zhao W L. A mitochondria-targeted turn-on fluorescent probe for the detection of glutathione in living cells[J]. Biosensors and Bioelectronics, 2016, 85: 164–170. DOI:10.1016/j.bios.2016.05.005 |
| [23] | Zhang J, Zhou J L, Dong X C, Zheng X, Zhao W L. A near-infrared BODIPY-based fluorescent probe for the detection of hydrogen sulfide in fetal bovine serum and living cells[J]. RSC Advances, 2016, 6(56): 51304–51309. DOI:10.1039/C6RA06952E |
| [24] | Shao X M, Kang R X, Zhang Y L, Huang Z T, Peng F F, Zhang J, Wang Y, Pan F C, Zhang W J, Zhao W L. Highly selective and sensitive 1 amino BODIPY-based red fluorescent probe for thiophenols with high off-to-on contrast ratio[J]. Analytical Chemistry, 2015, 87(1): 399–405. DOI:10.1021/ac5028947 |
| [25] | Zhang Y L, Shao X M, Wang Y, Pan F C, Kang R X, Peng F F, Huang Z T, Zhang W J, Zhao W L. Dual emission channels for sensitive discrimination of Cys/Hcy and GSH in plasma and cells[J]. Chemical Communications, 2015, 51(20): 4245–4248. DOI:10.1039/C4CC08687B |
| [26] | Schwarzer D D, Gritsch P J, Gaich T. Mimicking dimethylallyltryptophan synthase:experimental evidence for a biosynthetic cope rearrangement process[J]. Angewandte Chemie International Edition, 2012, 51(46): 11514–11516. DOI:10.1002/anie.201203586 |
| [27] | Peng B, Chen W, Liu C R, Rosser E W, Pacheco A, Zhao Y, Aguilar H C, Xian M. Fluorescent probes based on nucleophilic substitution-cyclization for hydrogen sulfide detection and bioimaging[J]. Chemistry-A European Journal, 2014, 20(4): 1010–1016. DOI:10.1002/chem.201303757 |
| [28] | Zhang D, Chen W, Miao Z R, Ye Y, Zhao Y F, King S B, Xian M. A reductive ligation based fluorescent probe for S-nitrosothiols[J]. Chemical Communications, 2014, 50(37): 4806–4809. DOI:10.1039/C4CC01288G |




