2. Henan Key Laboratory of Function-Oriented Porous Materials, College of Chemistry and Chemical Engineering, Luoyang Normal University, Luoyang 471934, Henan, P.R. China
2. 洛阳师范学院 化学化工学院 功能导向多孔材料河南省重点实验室, 河南 洛阳 471934
Phosphorescent transition metal complexes have attracted considerable attention for their application in organic light emitting devices(OLEDs), as they can make use of both singlet and triplet excitons resulting in high electroluminescence (EL) efficiency [1-3]. However the central ions of most phosphorescent complexes are noble metals, such as Ir(Ⅲ) and Pt(Ⅱ), which are expensive and have highly limited resources. In 1999, Ma and coworkers [4] reported the EL device with a phosphorescent copper(Ⅰ) complex as emitting layer for the first time, which offered a new type of phosphorescent emitter for OLEDs. Over the past decade, luminescent copper(Ⅰ) complexes have gained great progress in molecular structure designing, luminescence mechanism studying and EL performance, and so forth [5-14]. Due to the low cost and abundant resource of copper in comparison to other transition metals, luminescent copper(Ⅰ) complexes are seen as the promising alternative emitters for OLEDs.
In recent years, most studies focused on the homoleptic and heteroleptic cationic copper(Ⅰ) complexes [5-8, 15-23]. Nevertheless, it has been proven that neutral copper(Ⅰ) complexes have superior emission characteristics in contrast to their charged counterparts [24]. In addition, neutral copper(Ⅰ) complexes facilitate vacuum deposition and the balance of charges injection for OLEDs due to the lack of small mobile counterions.As we know, most neutral copper(Ⅰ) complexes showed excellent luminescence properties and some of them achieved high EL efficiencies [9-14, 25-30]. In the past, the diimine ligands with imidazole moiety are usually employed to synthesize cationic copper(Ⅰ) complexes [31-34], in fact, these ligands are suitable for neutral copper(Ⅰ) complexes, because the active H atom in imidazole unit is easy to be removed by strong bases resulting in anionic ligands [35, 36]. Herein, we used 2-(2-pyridyl)-1H-imidazole (Hpim) as the precursor of anionic ligand to synthesize a new neutral heteroleptic copper(Ⅰ) complex (pim) Cu(POP) (Scheme 1), which shows high stability and intense phosphorescence in solid state, where POP denotes bis[2-(diphenylphosphino)phenyl]ether.
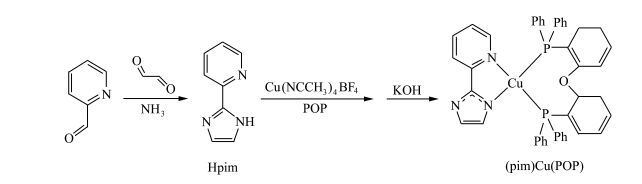
|
Scheme1 Synthetic route of (pim)Cu(POP) |
All reagents and solvents were purchased from commercial suppliers and used as received unless otherwise stated. 1HNMR, 13CNMR and 31PNMR spectra were recorded on a Bruker Avance500 spectrometer. Mass spectra were performed on a LCQ DecaXp Max instrument. Thermal gravimetric analyses were performed on a SII instrument TGA/DTA 6300 with a heating rate of 10 ℃/min under N2 atmosphere. Absorption spectra were recorded on a Hitachi U-3010 UV-Vis spectrophotometer. Steady-state emission spectra, emission lifetimes, and absolute emission quantum yields were recorded under N2 atmosphere on an Edinburgh FLS980 spectrometer equipped with a barium sulfate coated integrating sphere.
1.2 Synthesis of HpimAn ice-cold 2-pyridine carboxaldehyde (10 mmol, 1.1 g) in ethanol (10 mL) was added to 40% aqueous glyoxal (1.5 mL) in ethanol (5 mL) at 0 ℃, and then ice-cold concentrated aqueous NH3 (3.5 mL) was added without delay. The mixture was held at 0 ℃ for 1 h, and then was allowed to stir for 5 h at 25 ℃. After the ethanol was removed under reduced pressure, the resulting solution was extracted with ethyl ether for three times. The combined organic solution was dried over anhydrous MgSO4 and evaporated under reduced pressure. The crude product was purified by column chromatography (white powder, yield: 0.55 g, 38 %). 1HNMR (500 MHz, CDCl3): 11.46 (1H, s), 8.53 (1H, d, J=5.0), 8.23 (1H, d, J=8.0), 7.81(1H, t, J= 9.0), 7.27-7.17 (3H, m). ESI-MS m/z (M+-1): 144.1.
1.3 Synthesis of (pim)Cu(POP)At room temperature, Hpim (2 mmol, 0.29 g) in dichloromethane (10 mL) was slowly added to the dichloromethane solution(10 mL) of [Cu(CH3CN)4](BF4) (2 mmol, 0.63 g), and the mixture was stirred for 1 h. Then, POP (2 mmol, 1.08 g) in dichloromethane (10 mL) was slowly added and stirred for another 1 h. After the solution was filtered and evaporated to dryness, the residue was dissolved in methanol(25 mL). Solid state KOH (3 mmol, 0.17 g) was added to the solution, and the mixture was stirred for 6 h at room temperature. The precipitate was collected and washed with water for three times. After the precipitate was dissolved in 30 mL ethanol/dichloromethane (3/1), pure complex was obtained as pale green crystal by slowly evaporating the solvent (yield: 0.91 g, 61%). 1HNMR (500 MHz, CDCl3): 7.79 (1H, d, J= 5.0), 7.59 (1H, s), 7.24~7.22 (9H, m), 7.19~7.05(14H, m), 6.99 (3H, d, J=8.0), 6.91 (3H, t, J=7.5), 6.73~6.68(3H, m). 13CNMR (125 MHz, CDCl3): 158.55, 158.50, 158.45, 153.02, 148.12, 136.50, 134.34, 133.62, 132.74, 132.62, 132.50, 130.85, 129.32, 128.27, 128.23, 128.20, 125.88, 125.78, 125.69, 124.41, 120.23, 119.53. 31PNMR (200 MHz, CDCl3): -13.94.
1.4 X-ray CrystallographyCrystal data were collected on a Bruker SMART APEX-Ⅱ CCD diffractometer with Mo Kα radiation. The data were corrected for Lorentz-polarization factors as well as for absorption. Structures were solved by direct methods and refined by full-matrix least-squares methods on F2 with the SHELXL-97 program. All nonhydrogen atoms were refined anisotropically, while hydrogen atoms were placed in geometrically calculated positions. CCDC reference number of (pim)Cu(POP) is 1511744.
1.5 Density Functional CalculationsDensity functional theory (DFT) calculations were performed with Gaussian 09 software package, which employing the B3LYP functional with the LanL2DZ basis set for Cu and 6-31G* basis set for C, N, H, O, and P. Geometric parameters obtained from X-ray analyses were used as a starting point for geometry optimization in the ground state. Geometry optimization was carried out in the gas phase, and frequency calculation was performed to confirm the optimized structure to be true minimum value on the potential energy surface. The optimized geometry was used for time-dependent density functional (TD-DFT) calculations.
2 Results and Dicussiion 2.1 Synthesis and StructureThe diimine compound Hpim was prepared by the reported method [37]. The neutral copper(Ⅰ) complex (pim)Cu(POP) was synthesized via a two-step reaction as shown in Scheme 1. Firstly, [Cu(CH3CN)4](BF4) reacted with POP and Hpim in sequence at room temperature. The product of this step is the charged counterpart of (pim)Cu(POP), which was not purified as the intermediate. Then, the intermediate product was deprotonated with KOH in methanol to give the target complex. Pure complex was obtained as crystal by slowly evaporating the dichloromethane/ethanol solution of crude product. The structure of (pim)Cu(POP) was characterized by 1HNMR, 13CNMR and 31PNMR, and confirmed by X-ray crystallography. In solid state, (pim)Cu(POP) is very stable to air and has high thermal stability with a decomposition temperature (Td) of 303 ℃(Figure 1).
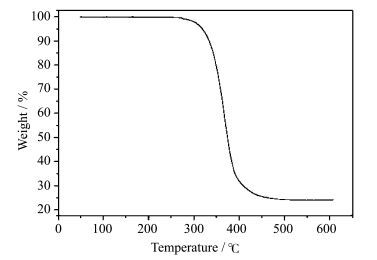
|
Fig.1 TGA curve of (pim)Cu(POP) |
The crystal structure of (pim)Cu(POP) was determined by single-crystal X-ray diffraction as shown in Figure 2. The complex crystallizes in monoclinic crystal system P21/c space group, and adopts a typical distorted tetrahedral geometry. The bond distances of Cu1-N1, Cu1-N3, Cu1-P1 and Cu1-P2 are 0.2006(4) nm, 0.2112(4) nm, 0.22511(13) nm and 0.22364(13) nm, respectively. The bond angles are N1-Cu1-N3=81.58(15)°, P1-Cu1-P2=111.84(15)°, N1-Cu1-P1=119.40(11)°, N3-Cu1-P1=106.12(10)°, N1-Cu1-P2=118.96(11)° and N3-Cu1-P2=114.20(11)°. These important bond distances and bond angles are similar to those of reported four-coordinate copper(Ⅰ) complexes [8-11, 22-25]. Analysis of the structural data shows that there is no π-π interaction or hydrogen bond in this complex.
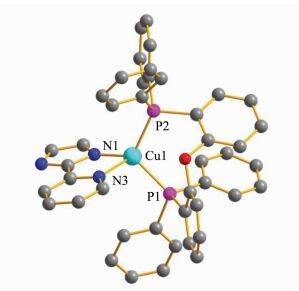
|
Fig.2 Crystal structure of (pim)Cu(POP) H atoms are omitted for clarity |
To gain insight into the electronic structure of (pim)Cu(POP), DFT calculations were performed using the geometric parameters obtained from X-ray analysis as the starting structure. Unlike most other copper(Ⅰ) complexes, the molecular orbitals (MOs) of (pim)Cu(POP) have very little metal character as shown in Figure 3. The highest occupied molecular orbital (HOMO) is almost concentrated on the anionic ligand pim, while the lowest unoccupied molecular orbital (LUMO) is dominated by the neutral ligand POP. TD-DFT calculations were performed to determine the lowest energy electronic transition in this complex, and the calculation results are summarized in Table 1. The lowest singlet state (S1) excitation is dominated by HOMO→LUMO transition, while the lowest triplet state(T1) excitation is mainly HOMO→LUMO + 4 transition. According to the DFT and TD-DFT calculation results, the lowest energy electronic transition of (pim)Cu(POP) is principally ligand-to-ligand charge transfer (LLCT) transition which mixed with minimal ligand-centered (LC) transition and metal-to-ligand charge transfer (MLCT) transition.
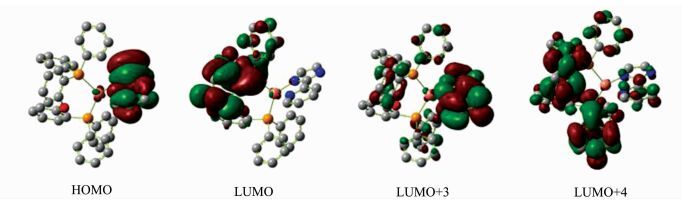
|
Fig.3 Calculated molecular orbitals of (pim)Cu(POP) |
| Table 1 The lowest energy transitions for (pim)Cu(POP) determined from TD-DFT calculations |
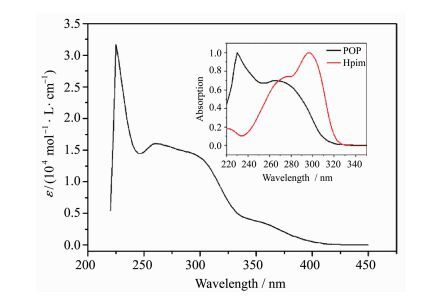
The UV-visible absorption spectrum of (pim)Cu(POP) was measured in ethanol solution as shown in Figure 4. Three intense high-energy absorption bands at around 225 nm (ε=3.16×104 mol-1·L·cm-1), 260 nm (ε=1.60×104 mol-1·L·cm-1) and 300 nm (ε=1.44×104 mol-1·L·cm-1) can be assigned to the π-π* transitions of ligands. The weak low-energy absorption band ranging from 340 nm to 415 nm (ε < 0.42×104 mol-1·L·cm-1) cannot be found in the absorption spectra of POP and Hpim(Figure 4 insert). According to the theory calculation results, the weak absorption band mainly originates from LLCT transition.
|
Fig.4 Absorption spectrum of (pim)Cu(POP) Insert is the absorption spectra of POP and Hpim |
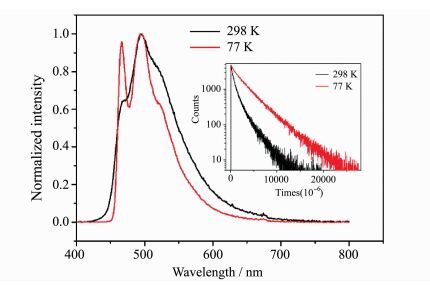
The complex (pim)Cu(POP) does not show detectable emission in solution at room temperature, which should be caused by Jahn-Teller distortion and solvent-induced exciplex, this luminescence quenching mechanism has been confirmed by several groups [38, 39]. In contrast, this complex shows intense emission in solid state at room temperature, the photoluminescence (PL) spectrum and photophysical data are given in Figure 5 and Table 2. The maximum emission peak locates at 495 nm, and two shoulder peaks appear at around 468 nm and 520 nm, respectively. The emission of (pim)Cu(POP) exhibits a moderate absolute quantum yield of 0.23 and a long lifetime of 855.8 μs.
|
Fig.5 Corrected emission spectra and emission decay behaviors (insert) of (pim)Cu(POP) in solid state at 298 K and 77 K |
| Table 2 Photophysical data of (pim)Cu(POP) |
It is well known, copper(Ⅰ) complexes probably give a thermally activated delayed fluorescence (TADF) at room temperature due to their small band gaps between S1 and T1. To understand the emissive states, low-temperature measurements were performed for (pim)Cu(POP) in solid state. As shown in Figure 5, the PL spectrum recorded at 77 K shows obvious fine structure, but do not red-shift compared to that recorded at 298 K. The emission lifetime was measured to be 2554.9 μs at 77 K. It can be found that the emission lifetime recorded at 77 K is only three times longer than that recorded at 298 K. The 77 K/298 K lifetime ratio is much smaller than those observed for most copper(Ⅰ) complexes with TADF [18, 29-32]. These experimental results indicate that the emission of (pim)Cu(POP) in solid state at room temperature is phosphorescence stemming from triplet state.
3 ConclusionIn summary, a new neutral copper(Ⅰ) complex with high stability were successfully prepared and characterized. The coordinate geometry of the complex is typical distorted tetrahedral configuration, and the bond lengths and bond angles are in the normal range. Unlike most other copper(Ⅰ) complexes, the lowest energy electronic transition of this complex is mainly LLCT transition in stead of MLCT transition. The complex shows intense emission with relative high efficiency and long lifetime in solid state. The emission can be identified as phosphorescence by comparing the photophysical data recorded at 298 K and 77 K.
| [1] | Xu H, Chen R, Sun Q. Recent progress in metal-organic complexes for optoelectronic applications[J]. Chemical Society Reviews, 2014, 43(10): 3259–3302. DOI:10.1039/C3CS60449G |
| [2] | Volz D, Wallesch M, Fléchon C. From iridium and platinum to copper and carbon: new avenues for more sustainability in organic light-emitting diodes[J]. Green Chemistry, 2015, 17(4): 1988–2011. DOI:10.1039/C4GC02195A |
| [3] | Chi Y, Tong B, Chou P T. Metal complexes with pyridylazolates: design, preparation and applications[J]. Coordination Chemistry Reviews, 2014, 281(1): 1–25. |
| [4] | Ma Y, Che C M, Chao H Y, Zhou X, Chan W H, Shen J. High luminescence gold(Ⅰ) and copper(Ⅰ) complexes with a triplet excited state for use in light-emitting diodes[J]. Advanced Materials, 1999, 11(10): 852–857. DOI:10.1002/(ISSN)1521-4095 |
| [5] | Cuttell D G, Kuang S M, Fanwick P E. Simple Cu(Ⅰ) complexes with unprecedented excited-state lifetimes[J]. Journal of the American Chemical Society, 2002, 124(1): 6–7. DOI:10.1021/ja012247h |
| [6] | Zhang Q, Zhou Q, Cheng Y, Wang L, Ma D, Jing X. Highly efficient green phosphorescent organic light-emitting diodes based on CuⅠ complexes[J]. Advanced Materials, 2004, 16(5): 432–436. DOI:10.1002/(ISSN)1521-4095 |
| [7] | Vorontsov Ⅱ, Graber T, Kovalevsky A Y. Capturing and analyzing the excited-state structure of a Cu(Ⅰ) phenanthroline complex by time-resolved diffraction and theoretical calculations[J]. Journal of the American Chemical Society, 2009, 131(18): 6566–6573. DOI:10.1021/ja900921p |
| [8] | Wada A, Zhang Q, Yasuda T. Efficient luminescence from a copper (Ⅰ) complex doped in organic light-emitting diodes by suppressing C-H vibrational quenching[J]. Chemical Communications, 2012, 48(43): 5340–5342. DOI:10.1039/c2cc31509b |
| [9] | Hsu C W, Lin C C, Chung M W. Systematic investigation of the metal-structure-photophysics relationship of emissive d10-complexes of group 11 elements: the prospect of application in organic light emitting devices[J]. Journal of the American Chemical Society, 2011, 133(31): 12085–12099. DOI:10.1021/ja2026568 |
| [10] | Hashimoto M, Igawa S, Yashima M. Highly efficient green organic light-emitting diodes containing luminescent three-coordinate copper (Ⅰ) complexes[J]. Journal of the American Chemical Society, 2011, 133(27): 10348–10351. DOI:10.1021/ja202965y |
| [11] | Gneuβ T, Leitl M J, Finger L H, Rau N. A new class of luminescent Cu(Ⅰ) complexes with tripodal ligands-TADF emitters for the yellow to red color range[J]. Dalton Transactions, 2015, 44(18): 8506–8520. DOI:10.1039/C4DT02631D |
| [12] | Leitl M J, Krylova V A, Djurovich P I, Thompson M E, Yersin H. Phosphorescence versus thermally activated delayed fluorescence. Controlling singlet-triplet splitting in brightly emitting and sublimable Cu(Ⅰ) compounds[J]. Journal of the American Chemical Society, 2014, 136(45): 16032–16038. DOI:10.1021/ja508155x |
| [13] | Hofbeck T, Monkowius U, Yersin H. Highly efficient luminescence of Cu(Ⅰ) compounds: thermally activated delayed fluorescence combined with short-lived phosphorescence[J]. Journal of the American Chemical Society, 2015, 137(1): 399–404. DOI:10.1021/ja5109672 |
| [14] | Deaton J C, Switalski S C, Kondakov D Y. E-type delayed fluorescence of a phosphine-supported Cu2(μ-NAr2)2 diamond core: harvesting singlet and triplet excitons in OLEDs[J]. Journal of the American Chemical Society, 2010, 132(27): 9499–9508. DOI:10.1021/ja1004575 |
| [15] | Gneuβ T, Leitl M J, Finger L H, Yersin H, Sundermeyer J. A new class of deep-blue emitting Cu(Ⅰ) compounds-effects of counter ions on the emission behavior[J]. Dalton Transactions, 2015, 44(46): 20045–20055. DOI:10.1039/C5DT03065J |
| [16] | Coppens P, Sokolow J, Trzop E. On the biexponential decay of the photoluminescence of the two crystallographically-independent molecules in crystals of [Cu(Ⅰ)(phen)(PPh3)2][BF4][[J]. Journal of Physics Chemistry Letters, 2013, 4(4): 579–582. DOI:10.1021/jz400013b |
| [17] | Wang Z, Zheng C, Wang W, Xu C, Ji B, Zhang X. Synthesis, structure, and photophysical properties of two four-coordinate CuI-NHC complexes with efficient delayed fluorescence[J]. Inorganic Chemistry, 2016, 55(5): 2157–2164. DOI:10.1021/acs.inorgchem.5b02546 |
| [18] | Liang D, Chen X L, Liao J Z, Hu J Y, Jia J H, Lu C Z. Highly efficient cuprous complexes with thermally activated delayed fluorescence for solution-processed organic light-emitting devices[J]. Inorganic Chemistry, 2016, 55(15): 7467–7475. DOI:10.1021/acs.inorgchem.6b00763 |
| [19] | Elie M, Sguerra F, Meo F D. Designing NHC-copper(Ⅰ) dipyridylamine complexes for blue light-emitting electrochemical cells[J]. ACS Applied Materials & Interfaces, 2016, 8(23): 14678–14691. |
| [20] | Chai W, Hong M, Song L. Three reversible polymorphic copper(Ⅰ) complexes triggered by ligand conformation: insights into polymorphic crystal habit and luminescent properties[J]. Inorganic Chemistry, 2015, 54(9): 4200–4207. DOI:10.1021/ic502709b |
| [21] | Marion R, Sguerra F, Meo F D. NHC copper(Ⅰ) complexes bearing dipyridylamine ligands: synthesis, structural, and photoluminescent studies[J]. Inorganic Chemistry, 2014, 53(17): 9181–9191. DOI:10.1021/ic501230m |
| [22] | Femoni C, Muzzioli S, Palazzi A. New tetrazole-based Cu(Ⅰ) homo-and heteroleptic complexes with various P∧P ligands: synthesis, characterization, redox and photophysical properties[J]. Dalton Transactions, 2013, 42(4): 997–1010. DOI:10.1039/C2DT32056H |
| [23] | Czerwieniec R, Kowalski K, Yersin H. Highly efficient thermally activated fluorescence of a new rigid Cu(Ⅰ) complex [Cu(dmp)(phanephos)]+[J]. Dalton Transactions, 2013, 42(27): 9826–9830. DOI:10.1039/c3dt51006a |
| [24] | Krylova V A, Djurovich P I, Whited M T, Thompson M E. Synthesis and characterization of phosphorescent three-coordinate Cu(Ⅰ)-NHC complexes[J]. Chemical Communications, 2010, 46(36): 6696–6698. DOI:10.1039/c0cc01864c |
| [25] | Krylova V A, Djurovich P I, Aronson J W. Structural and photophysical studies of phosphorescent three-coordinate copper(Ⅰ) complexes supported by an N-heterocyclic carbene ligand[J]. Organometallics, 2012, 31(22): 7983–7993. DOI:10.1021/om300656v |
| [26] | Krylova V A, Djurovich P I, Conley B L. Control of emission colour with N-heterocyclic carbene (NHC) ligands in phosphorescent three-coordinate Cu(Ⅰ) complexes[J]. Chemical Communications, 2014, 50(54): 7176–7179. DOI:10.1039/C4CC02037E |
| [27] | Kang L, Chen J, Teng T, Chen X L, Yu R, Lu C Z. Experimental and theoretical studies of highly emissive dinuclear Cu(Ⅰ) halide complexes with delayed fluorescence[J]. Dalton Transactions, 2015, 44(30): 11649–11659. |
| [28] | Okano Y, Ohara H, Kobayashi A, Yoshida M, Kato M. Systematic introduction of aromatic rings to diphosphine ligands for emission color tuning of dinuclear copper(Ⅰ) iodide complexes[J]. Inorganic Chemistry, 2016, 55(11): 5227–5236. DOI:10.1021/acs.inorgchem.6b00161 |
| [29] | Czerwieniec R, Yersin H. Diversity of copper(Ⅰ) complexes showing thermally activated delayed fluorescence: basic photophysical analysis[J]. Inorganic Chemistry, 2015, 54(9): 4322–4327. DOI:10.1021/ic503072u |
| [30] | Igawa S, Hashimoto M, Kawata I. Highly efficient green organic light-emitting diodes containing luminescent tetrahedral copper(Ⅰ) complexes[J]. Journal of Materials Chemistry C, 2013, 1(3): 542–551. DOI:10.1039/C2TC00263A |
| [31] | Zhang L, Li B, Su Z. Realization of high-energy emission from [Cu(N-N)(P-P)]+ complexes for organic light-emitting diode applications[J]. The Journal of Physical Che-mistry C, 2009, 113(31): 13968–13973. DOI:10.1021/jp903774g |
| [32] | Jia W L, McCormick T, Tao Y, Lu J P, Wang S. New phosphorescent polynuclear Cu(Ⅰ) compounds based on linearand star-shaped 2-(2′-Pyridyl)benzimidazolyl derivatives: syntheses, structures, luminescence, and electroluminescence[J]. Inorganic Chemistry, 2005, 44(16): 5706–5712. DOI:10.1021/ic0504893 |
| [33] | Si Z, Li J, Li B, Liu S, Li W. High light electroluminescence of novel Cu(Ⅰ) complexes[J]. Journal of Luminescence, 2009, 129(3): 181–186. DOI:10.1016/j.jlumin.2008.09.014 |
| [34] | Zhang D. Novel green-emitting copper(Ⅰ) complexes with electron donors incorporated ligands: synthesis, photophysical properties, and electroluminescence performances[J]. Journal of Luminescence, 2010, 130(8): 1419–1424. DOI:10.1016/j.jlumin.2010.03.005 |
| [35] | Min J, Zhang Q, Sun W, Cheng Y, Wang L. Neutral copper(Ⅰ) phosphorescent complexes from their ionic counterparts with 2-(2′-quinolyl)benzimidazole and phosphine mixed ligands[J]. Dalton Transactions, 2011, 40(3): 686–693. DOI:10.1039/C0DT01031F |
| [36] | Bergmann L, Friendrichs J, Mydlak M. Outstanding luminescence from neutral copper(Ⅰ) complexes with pyridyl-tetrazolate and phosphine ligands[J]. Chemical Communications, 2013, 49(58): 6501–6503. DOI:10.1039/c3cc42280a |
| [37] | Wang R, Xiao J C, Twamley B, Shreeve J M. Efficient heck reactions catalyzed by a highly recyclable palladium(Ⅱ) complex of a pyridyl-functionalized imidazolium-based ionic liquid[J]. Organic & Biomolecular Chemistry, 2007, 5(4): 671–678. |
| [38] | Scaltrito D V, Thompson D W, O′Callaghan J A, Meyer G J. MLCT excited states of cuprous bis-phenanthroline coordination compounds[J]. Coordination Chemistry Reviews, 2000, 208(1): 243–266. DOI:10.1016/S0010-8545(00)00309-X |
| [39] | Armaroli N. Photoactive mono-and polynuclear Cu(Ⅰ)-phenanthrolines. A viable alternative to Ru(Ⅱ)-polypyridines[J]. Chemical Society Reviews, 2001, 30(2): 113–124. DOI:10.1039/b000703j |




