铝是我们日常生活中广泛接触的金属元素之一,如药品、包装材料和灶具等都含有铝元素。当体内铝元素过量时,不仅会影响动植物的生长,还会破坏人体的神经系统进而引起如阿尔茨海默氏病、肌萎缩性侧索硬化症和帕金森等疾病[1, 2]。因此,检测环境及生物样品中的Al3+含量具有特殊意义。在众多检测铝离子的方法中,荧光光谱法具有方法简单、选择性好、灵敏度高、快速和成本低等优点,因而近年来检测铝离子的荧光传感器受到了人们的广泛关注[3-23]。然而,与Al3+弱的结合能力及强的水合作用使得高选择性、高灵敏度的Al3+荧光探针的发展受到了限制。与其它过渡金属离子相比,用于检测Al3+的荧光探针不多,且适用于缓冲水体系检测的较少,能用于细胞检测的体系更少。因此,研制简单经济、水溶性好、能用于细胞成像检测Al3+的荧光探针具有重要的应用价值。
由于合成步骤简单且具有良好的配位能力,含有N和O的希夫碱衍生物常被设计为荧光探针,用于检测金属离子。本文以2-羟基-1-萘甲醛和2-氨甲基吡啶为原料合成了希夫碱荧光探针(L)。已有文献报道该探针分子可与Cu2+、Zn2+、Sn2+等金属离子配位[24-28]。文献[28]还利用该探针分子建立了在中性水介质中识别检测Zn2+的荧光方法。考虑到该探针分子中富电子的硬碱氮、氧原子可与作为硬酸的Al3+结合,且探针分子和金属离子的配位作用受pH值影响较大,我们深入研究了L探针分子在不同pH下对金属离子的光谱响应。实验结果表明,L在弱酸性缓冲溶液中能选择性检测金属离子Al3+,且体系具有高灵敏度。本文通过实验详细探讨了探针L与Al3+的结合模式及响应机理,并将建立的分析方法应用于实际样品(细胞及水样)中Al3+的识别与检测。
1 实验部分 1.1 试剂与仪器试剂:2-羟基-1-萘甲醛(98%,萨恩化学技术有限公司,上海);2-氨甲基吡啶(阿拉丁,上海);其它试剂均为分析纯,不需进一步纯化;金属离子为相应的硝酸盐配制;实验用水为二次蒸馏水。
仪器:紫外吸收光谱仪(UV-2450,岛津,日本);荧光光谱仪(LS-55,PerkinElmer,美国),激发波长为390 nm,激发和发射狭缝分别为7 nm和5 nm;红外光谱仪(SHIMADZU 8400S,Nicolet, Waltham, 日本);核磁共振仪(DRX-600,Fällenden, Switzerland);高效液相质谱仪(Bru-ker microTOF-Q Ⅲ);pH计(pHS-3C,上海奥豪仪器有限公司);Olympus FV1000共聚焦显微镜(日本)。
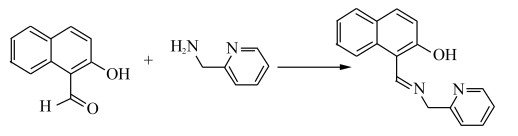
1.2 实验方法探针L的合成路线如Scheme 1所示。
|
Scheme 1 L的合成 Scheme1 Synthesis of L |
2-氨甲基吡啶(0.22 g,2 mmol)和2-羟基-1-萘甲醛(0.34 g,2 mmol)在15 mL甲醇中回流反应3 h,冷却至室温,旋干,加少量氯仿和乙醚,析出固体,抽滤得到淡黄色固体L,产率为80%。
IR νmax (KBr):3433.45, 1624.79, 1546.94, 1455.92, 1384.30, 1337.40, 1309.01, 1249.38, 868.23, 765.06 cm-1。1HNMR (DMSO-d6): 14.30 (s, 1H, OH), 9.30 (d, 1H, J=12.00 Hz, HC=N), 8.60 (d, J=6.00 Hz, 1H, ArH), 8.11 (d, J=6.00 Hz, 1H, ArH), 7.85 (t, J=6.00 Hz, 1H, ArH), 7.75 (d, J=6.00 Hz, 1H, ArH), 7.65(d, J=6.00 Hz, 1H, ArH), 7.46(t, J=6.00 Hz, 2H, ArH), 7.36 (t, J=6.00 Hz, 1H, ArH), 7.22 (t, J=6.00 Hz, 1H, ArH), 6.74 (d, J=6.00 Hz, 1H, ArH), 5.00 (s, 2H, CH2). 13CNMR (DMSO-d6): 177.34, 160.36, 157.18, 149.61, 137.69, 134.73, 129.40, 128.44, 125.82, 125.72, 123.32, 122.81, 122.40, 119.03, 106.55, 56.62。HRMS (ESI+) [L+H]+, calculated for C17H14N2O 263.1184,found 263.1179。
光谱测定:将探针L溶于DMSO溶剂中,制得1×10-3 mol/L的储备液,配制10-2 mol/L的金属离子储备液用于整个实验过程中。Al3+滴定实验过程为:取20 μL的探针L于比色管中,加入一定量的Al3+,加入100 μL pH 4.2的六次甲基四胺-HCl缓冲溶液,用DMSO:H2O=4:1的溶剂定容至5 mL,放置5 min后进行光谱的测定。
细胞成像:使用人体SiHa癌细胞进行体外细胞实验。首先在37 ℃下将SiHa癌细胞在含5%CO2、10%的胎牛血清培养基中培育。然后用0.1 mol/L无菌PBS缓冲液清洗细胞,将细胞在50 μL的L探针溶液中孵育30 min。再用PBS缓冲液(pH 4.5)清洗细胞,加入150 μL Al3+溶液,继续孵育30 min。最后,用PBS缓冲液清洗细胞3次,在Olympus FV1000共聚焦显微镜下以40×物镜得到荧光细胞图像(λex=405 nm)。
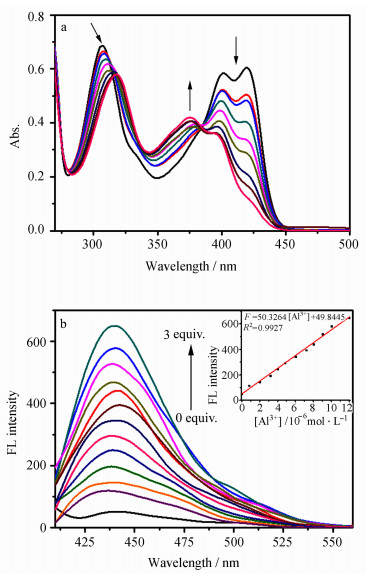
2 结果与讨论 2.1 Al3+对L紫外和荧光光谱的影响在pH 4.2的DMSO-H2O(4:1, V/V)的溶液中测定了Al3+对L光谱的影响。如图 1a所示,L在307 nm、401 nm和420 nm处有特征吸收峰,摩尔吸光系数分别为1.26×104 L·mol-1·cm-1、1.11×104 L·mol-1·cm-1和1.17×104 L·mol-1·cm-1。其中307 nm处为分子内π-π*跃迁产生的吸收峰,401 nm和420 nm为分子内n-π*跃迁产生的吸收峰[10, 26, 27]。随着Al3+浓度的增加(0~8 equiv.),L在307 nm的吸收峰红移至318 nm,同时,401 nm和420 nm处的吸收峰强度逐渐降低并在375 nm处产生一个新的吸收峰,这说明希夫碱L和Al3+之间发生了络合作用。此外,在377 nm处产生的等吸收点表明L与Al3+形成了一种新的络合物。
|
图 1 在DMSO/H2O (4:1, V/V, pH 4.2)体系中,(a)不同浓度的Al3+(0~8 equiv.)对L (60 μmol/L)紫外吸收光谱的影响;(b)不同浓度的Al3+(0~3 equiv.)对L (4 μmol/L)荧光光谱的影响,插图为荧光强度与Al3+浓度的线性关系图 Fig.1 (a) Absorption spectra of L (60 μmol/ L) in the presence of different concentrations of Al3+ (up to 8 equiv.) and (b) fluorescence spectra of L (4 μmol/L) in the presence of different concentrations of Al3+ (up to 3 equiv.) in DMSO/H2O (4:1, V/V, pH 4.2) Inset: the fluorescence intensity of L as a function of Al3+ ion concentration |
图 1b为不同浓度Al3+对L荧光光谱的影响。在390 nm光的激发下,因分子中C=N的异构化反应使得L分子本身在440 nm处的发射很弱。随着Al3+ (0~3 equiv.)的加入,体系在438 nm处的发射峰逐渐增强。这种变化是由于L与Al3+的结合阻止了C=N的异构化,同时使体系刚性增强而引起螯合型荧光增强(CHEF)[3, 7, 17-20]。
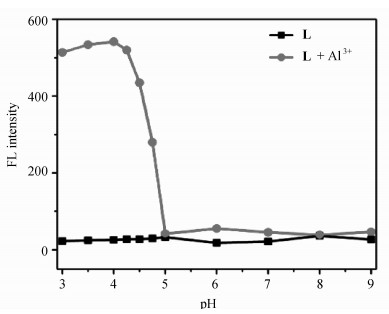
2.2 L对Al3+的荧光测定首先, 考查pH对L及L -Al3+荧光强度的影响。如图 2所示,L本身荧光很弱,在pH 3.0~9.0范围内,几乎不受pH的影响。相比之下,L -Al3+体系受pH影响较大。在pH值3.0~4.5范围内,体系的荧光强度最大并保持稳定,pH大于4.5时,荧光强度就明显降低。其原因可能是当pH很小时,L中的氮原子质子化,不利于L和Al3+的络合,而在较大pH时,Al3+会在溶液中形成沉淀。因此, 本文我们选用pH为4.2的六次甲基四胺-HCl缓冲溶液来调节体系的pH。
|
图 2 pH对L和L -Al3+体系荧光强度的影响 Fig.2 Effect of pH on fluorescence intensity of the L -Al3+complex in DMSO/H2O (4:1, V/V) [L] = 4 μmol/L |
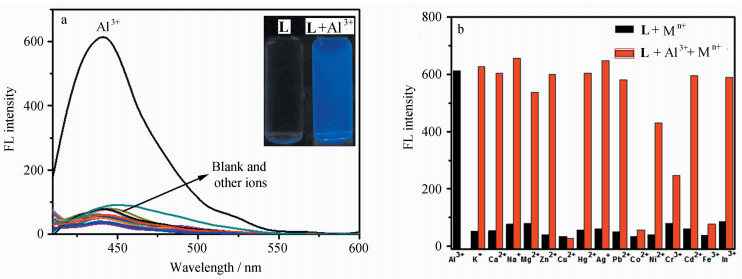
接下来考查了L对金属离子的选择性,实验结果如图 3a所示。在相同的实验条件下,L中只有加入Al3+后,荧光才明显增强。如图 3a插图所示,在365 nm紫外灯照射下,L中加入Al3+后,体系发出强的蓝色荧光,而加入其他离子,荧光几乎没有发生变化。此外,如图 3b所示,在L -Al3+体系中有其他共存离子时,除Cu2+、Fe3+和Co2+外,其他离子都不会影响L -Al3+体系的荧光强度,表明L在大多数离子共存时可选择性测定Al3+。根据荧光滴定(图 1b插图)结果,计算得该探针对铝离子的检测限为8.05×10-8 mol/L(信噪比为3:1),小于世界卫生组织规定的饮用水中Al3+的含量,因此可检测饮用水中的Al3+。此探针的灵敏度、检测限相当或优于目前所报道的大多数检测Al3+的希夫碱荧光探针[5, 7, 8, 11, 12, 16]。
|
图 3 (a) L对金属离子的选择性;(b)共存离子对L -Al3+体系的影响 Fig.3 (a) Fluorescence spectra changes of L (4 μmol/L) in the presence of various metal ions (12 μmol/L) in DMSO/H2O (4:1, V/V, pH 4.2); (b) Fluorescence intensities of L (4 μmol/L) at 438 nm (I438) upon addition of Al3+ in the presence of interfered metal ions (12 μmol/L) |
如图 4a所示,等摩尔连续变换法测得L与Al3+的结合比为1:1。如图 4b所示,通过B-H方程[29]计算得到L与Al3+的结合常数为6.3×104 L·mol-1。

|
图 4 (a) L与Al3+的结合比测定曲线;(b) Benesi-Hildebrand双倒数曲线 Fig.4 (a) Job's plot for L and Al3+ complexation in DMSO/H2O (4:1, V/V, pH 4.2), the total concentration of L and Al3+ is 10 μmol/L; (b) Benesi-Hildebrand plot from fluorescence titration data of L with Al3+ |
我们进一步通过高分辨质谱证明了L与Al3+的结合比为1:1。如图 5所示,峰值306.1711为[L + Al-H + H2O]的分子离子峰(理论计算值为m/z, 306.0949)。
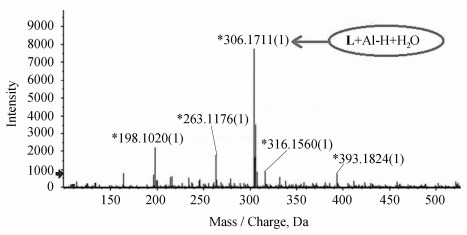
|
图 5 L与Al3+的高分辨质谱图 Fig.5 ESI-mass spectrum of L (1.0×10-5 mol·L-1) upon addition of 1.5 equiv. of Al3+ in MeOH |
最后, 通过核磁滴定考查L与Al3+的结合模式。如图 6所示,随着Al3+的加入,L中酚羟基在11.09处的质子峰(H1)逐渐消失,表明L与Al3+结合后使羟基发生了去质子化。此外,L分子中—CH=N质子峰(H2)、亚甲基质子峰(H3)和吡啶环上的质子峰(H4~H7)均向低场位移,表明Al3+与L中吡啶环上的—N、亚胺基—N和酚羟基的—O进行了结合。
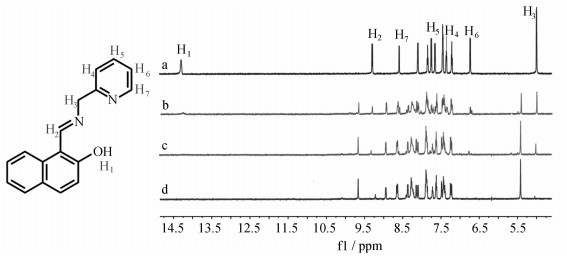
|
图 6 不同浓度Al3+对L核磁共振谱的影响 Fig.6 1HNMR spectra of L in the presence of different concentrations of Al (NO3)3·9H2O in d6-DMSO: (a) L only; (b) L + Al3+(0.25 equiv.); (c) L + Al3+(0.5 equiv.); (d) L + Al3+(1 equiv.) |
为探索该探针在实际中的应用,本文初步进行了对自来水、太原晋阳湖及山西大学令德湖水中Al3+的检测,实验结果如表 1所示,可知探针能够检测加标Al3+的浓度,而且具有较好的回收率,表明该方法可以用于实际水样品中Al3+的检测。
| 表 1 水样中Al3+的检测 Table 1 Determination of Al3+ in water samples |
另外,通过荧光共聚焦成像,证明了L在生物样品中测定Al3+具有潜在价值,实验结果如图 7所示。人体SiHa癌细胞在50 μmol/L的探针L中培养30 min后无荧光产生,加入150 μmol/L Al3+继续培养后,可观察到很强的蓝色荧光。结果显示SiHa癌细胞在整个实验过程中保持正常的形态特征,表明该探针基本无毒,可应用于体内细胞中Al3+的检测。
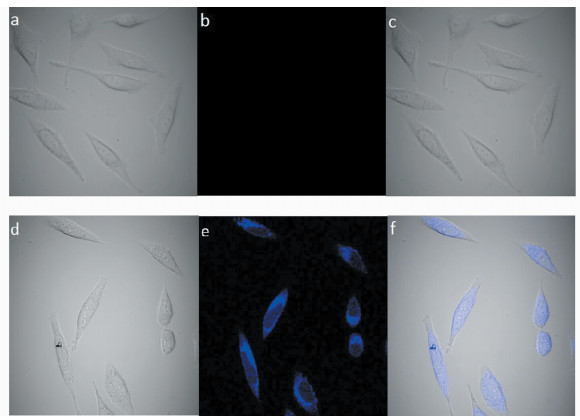
|
图 7 L与L -Al3+在细胞中的荧光成像图 Fig.7 Confocal fluorescence and bright-field images of Human SiHa cancer cells stained with L (a-c) and with both L and Al3+(d-f) (Left) bright field image; (middle) fluorescence image; (right) merged image [L]=50 μmol/L, [Al3+] =150 μmol/L (λex=405 nm) |
本文合成了一种在弱酸性缓冲水溶液中识别检测Al3+的希夫碱荧光探针(L)。该探针与Al3+以1:1结合后,分子内C=N旋转受到限制,刚性增强,从而使体系荧光显著增强。L对Al3+的检测限为80.5 nmol/L,远低于世界卫生组织规定的饮用水中Al3+的上限值(7.41 μmol/L)。该探针合成简单、检测限低,能在水体系和细胞内实现对Al3+的检测,具有潜在的应用价值。
| [1] | Altschuler E. Aluminum-containing antacids as a cause of idiopathic Parkinson's disease[J]. Medical Hypotheses, 1999, 53(1): 22–23. DOI:10.1054/mehy.1997.0701 |
| [2] | Kepp K P. Bioinorganic chemistry of Alzheimer's disease[J]. Chemical Reviews, 2012, 112(10): 5193–5239. DOI:10.1021/cr300009x |
| [3] | Kim S, Noh J Y, Kim K Y, Kang H K, Nam S W, Kim S H, Park S, Kim C, Kim J. Salicylimine-based fluorescent chemosensor for aluminum ions and application to bioima-ging[J]. Inorganic Chemistry, 2012, 51(6): 3597–3602. DOI:10.1021/ic2024583 |
| [4] | Shi X Y, Wang H, Han T Y, Feng X, Tong B, Shi J B, Zhi J G, Dong Y P. A highly sensitive, single selective, real-time and "turn-on" fluorescent sensor for Al3+ detection in aqueous media[J]. Journal of Materials Chemistry, 2012, 22(36): 19296–19302. DOI:10.1039/c2jm33393g |
| [5] | Helal A, Kim S H, Kim H S. A highly selective fluorescent turn-on probe for Al3+ via Al3+-promoted hydrolysis of ester[J]. Tetrahedron, 2013, 69(30): 6095–6099. DOI:10.1016/j.tet.2013.05.062 |
| [6] | Das S, Sahana A, Banerjee A, Lohar S, Safin D A, Babashkina M G, Bolte M, Garcia Y, Hauli I, Mukhopadhyay S K, Das D. Ratiometric fluorescence sensing and intracellular imaging of Al3+ ions driven by an intramolecular excimer formation of a pyrimidine-pyrene scaffold[J]. Dalton Transactions, 2013, 42(14): 4757–4763. DOI:10.1039/c3dt32908a |
| [7] | Gupt V K, Singh A K, Kumawat L K. Thiazole Schiff base turn-on fluorescent chemosensor for Al3+ ion[J]. Sensors and Actuators B:Chemical, 2014, 195(5): 98–108. |
| [8] | Peng L, Zhou Z J, Wang X Y, Wei R R, Li K, Xiang Y, Tong A J. A ratiometric fluorescent chemosensor for Al3+ in aqueous solution based on aggregation-induced emission and its application in live-cell imaging[J]. Analytica Chi-mica Acta, 2014, 829(6): 54–59. |
| [9] | Wang J F, Li Y B, Patel N G, Zhang G, Zhou D M, Pang Y. A single molecular probe for multi-analyte (Cr3+, Al3+ and Fe3+) detection in aqueous medium and its biological application[J]. Chemical Communications, 2014, 50(82): 12258–12261. DOI:10.1039/C4CC04731A |
| [10] | Xia S, Xiao S Y, Hong Q Q, Zou J R, Yang S, Zhang M X, Zuo H. A novel sensitive fluorescent turn-on probe for rapid detection of Al3+ and bioimaging[J]. RSC Advances, 2015, 5(7): 5244–5249. DOI:10.1039/C4RA14177F |
| [11] | Wang H H, Wang B, Shi Z H, Tang X L, Dou W, Han Q X, Zhang Y G, Liu W S. A two-photon probe for Al3+ in aqueous solution and its application in bioimaging[J]. Biosensors and Bioelectronics, 2015, 65: 91–96. DOI:10.1016/j.bios.2014.10.018 |
| [12] | Das S, Goswami S, Aich K, Ghoshal K, Quah C K, Bhattacharyya M, Fun H K. ESIPT and CHEF based highly sensitive and selective ratiometric sensor for Al3+ with imaging in human blood cells[J]. New Journal of Chemistry, 2015, 39(11): 8582–8587. DOI:10.1039/C5NJ01468A |
| [13] | Lee J J, Park G J, Kim Y S, Lee S Y, Lee H J, Noh I, Kim C. A water-soluble carboxylic-functionalized chemosensor for detecting Al3+ in aqueous media and living cells:experimental and theoretical studies[J]. Biosensors and Bioelectronics, 2015, 69: 226–229. DOI:10.1016/j.bios.2015.02.038 |
| [14] | Kumar V, Kumar A, Diwan U, Srivastava S K, Upadhyay K K. Salicylideneimines as efficient dual channel emissive probes for Al3+:harnessing ESIPT and ICT processes[J]. Sensors and Actuators B, 2015, 207: 650–657. DOI:10.1016/j.snb.2014.10.068 |
| [15] | Liang C S, Bu W H, Li C L, Men G W, Deng M Y, Jiangyao Y K, Sun H C, Jiang S M. A highly selective fluorescent sensor for Al3+ and the use of the resulting complex as a secondary sensor for PPi in aqueous media:its applicability in live cell imaging[J]. Dalton Transactions, 2015, 44(25): 11352–11359. DOI:10.1039/C5DT00689A |
| [16] | Kumar J, Sarma M J, Phukan P, Das D K. A new simple Schiff base fluorescence "on" sensor for Al3+ and its living cell imaging[J]. Dalton Transactions, 2015, 44(10): 4576–4581. DOI:10.1039/C4DT03932G |
| [17] | Sinha S, Chowdhury B, Ghosh P. A highly sensitive ESIPT-based ratiometric fluorescence sensor for selective detection of Al3+[J]. Inorganic Cheistry, 2016, 55(18): 9212–9220. DOI:10.1021/acs.inorgchem.6b01170 |
| [18] | Torawane P, Tayade K, Bothra S, Sahoo S K, Singh N, Borse A, Kuwar A. A highly selective and sensitive fluorescent 'turn-on' chemosensor for Al3+ based on C=N isomerisation mechanism with nanomolar detection[J]. Sensors and Actuators B, 2016, 222: 562–566. DOI:10.1016/j.snb.2015.08.104 |
| [19] | Shyamal M, Mazumdar P, Maity S, Sahoo G P, Salgado-Moraán G, Misra A. Pyrene scaffold as real-time fluorescent turn-on chemosensor for selective detection of trace-level Al(Ⅲ) and its aggregation-induced emission enhancement[J]. Journal of Physical Chemistry A, 2016, 120(2): 210–220. DOI:10.1021/acs.jpca.5b09107 |
| [20] | Li W, Tian X H, Huang B, Li H J, Zhao X Y, Gao S, Zheng J, Zhang X Z, Zhou H P, Tian Y P, Wu J Y. Triphenylamine-based Schiff bases as the high sensitive Al3+ or Zn2+ fluorescence turn-on probe:mechanism and application in vitro and in vivo[J]. Biosensors and Bioelectro-nics, 2016, 77: 530–536. DOI:10.1016/j.bios.2015.09.059 |
| [21] | Hossain S M, Singh K, Lakma A, Pradhan R N, Singh A K. A schiff base ligand of coumarin derivative as an ICT-based fluorescence chemosensor for Al3+[J]. Sensors and Actuators B, 2017, 239: 1109–1117. DOI:10.1016/j.snb.2016.08.093 |
| [22] | Kumawata L K, Asifb M, Gupta V K. Dual ion selective fluorescence sensor with potential applications in sample monitoring and membrane sensing[J]. Sensors and Actuators B, 2017, 241: 1090–1098. DOI:10.1016/j.snb.2016.10.031 |
| [23] | Tang L J, Ding S L, Zhong K L, Hou S H, Bian Y J, Yan X M. A new 2-(2'-hydroxyphenyl)quinazolin-4(3H)-one derived acylhydrazone for fluorescence recognition of Al3+[J]. Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy, 2017, 174: 70–74. DOI:10.1016/j.saa.2016.11.026 |
| [24] | You Z L, Zhu H L. Syntheses, crystal structures, and antibacterial activities of four Schiff base complexes of copper and zinc[J]. Zeitschrift fuer Anorganische und Allgemeine Chemie, 2004, 630(15): 2754–2760. DOI:10.1002/(ISSN)1521-3749 |
| [25] | Tahereh S, Zahra S P. Synthesis and spectroscopic studies of new organotin(Ⅳ) complexes with tridentate N-and O-donor Schiff bases[J]. Journal of Coordination Chemistry, 2009, 62(23): 3837–3844. DOI:10.1080/00958970903180103 |
| [26] | Pulimamidi R R, Addla S. 2-Hydroxynaphthalene-1-carbaldehyde-and 2-(aminomethyl)pyridine-based Schiff base CuⅡ complexes for DNA binding and cleavage[J]. Chemistry and Biodiversity, 2012, 9(10): 2262–2281. DOI:10.1002/cbdv.v9.10 |
| [27] | Zahra K, Hadi A R, Valiollah M, Mehdi S, Majid M, Sharam T, Iraj M B. Synthesis, characterization, crystal structure, DNA-and HAS-binding studies of a dinuclear Schiff base Zn(Ⅱ) complex derived from 2-hydroxynaphtaldehyde and 2-picolylamine[J]. Journal of Molecular Structure, 2015, 1096: 110–120. DOI:10.1016/j.molstruc.2015.04.033 |
| [28] | Wu K, Gao Y T, Yu F Q, Jiang J H, Guo J X, Han Y F. A facile fluorescent chemosensor based on naphthalene-derived Schiff base for zinc ions in aqueous solution[J]. Analytical Methods, 2014, 6: 3560–3563. DOI:10.1039/C4AY00431K |
| [29] | Barra M, Bohne C, Scaiano J C. Effect of cyclodextrin complexation on the photochemistry of xanthone absolute measurement of the kinetics for triplet-state exit[J]. Journal of the American Chemical Society, 1990, 112(22): 8075–8079. DOI:10.1021/ja00178a034 |




