2. School of Chemical Engineering and Technology, Tianjin University, Tianjin 300350, P. R. China;
3. University of Chinese Academy of Sciences, Beijing 100049, P. R. China
2. 天津大学 化学院, 天津 300350;
3. 中国科学院大学, 北京 100049
The atmosphere concentration of carbon dioxide (CO2) has been accumulating, causing issues of global warming[1].The electrochemcial CO2 reduction reaction (CO2RR) is a promising approach of converting CO2 to fuels or chemicals with renewable energy[2].One of the research focus is to develop catalysts with excellent catalytic activity and high selectivity for CO2RR[3]. Various metal based catalysts including Cu[4], Au[5], Ag[6], Co[7] or metal oxide catalysts including Cu2O[8, 9], ZnO[10] and SnO[11], etc., have been studied in the last few years. However, high overpotential, poor stability and low selectivity still hinder the application of CO2RR[12].Developing inexpensive, selective and stable catalysts with low overpotential is therefore an important requirement[13].
Niobium oxides[14, 15] and nitrides[16] have decent physical properties. The combination of graphitic carbon nitride (g-C3N4) with Nb2O5 can degrade organic contaminants[17]. Niobium nitride Nb4N5 is a good electrode material for supercapacitors to improve its capacity and stability[18]. Niobium nitride is also used as a novel anode for lithium ion battery[19].We reported on the application of tantalum in the catalytic reduction of CO2[20]. However, as the same family element with Ta, studies of niobium in electrocatalytic reduction of CO2 have been seldom reported.
In this work, we have prepared different types of niobium nitrides and niobium oxides to explore the catalytic property on CO2 reduction. In CO2 electrochemical reduction, carbon loaded Nb4N5 by NH3 treatment at 700 ℃ (denoted as Nb4N5-700) exhibited current density of 4.7 mA·cm-2 with the Faradaic efficiency of 57% for CO production in CO2 saturated 0.5 mol·L-1 NaCl electrolyte at -0.83 V vs RHE. Based on analysis of electrochemical properties, the extent of N doping into Nb4N5 played an important role in CO2RR.
1 Experimental 1.1 MaterialsAll the chemicals were of analytical reagent grade. Niobium chloride (NbCl5, 99.95%, Energy Chemical), ethanol (C2H5OH, ≥99.7%, Beijing Chemical Works), carbon black (XC-72R), ammonia solution (NH3·H2O, 5%, Beijing Chemical Works), ammonia gas (NH3, 99.999%, JINGHUI GAS), argon (Ar, 99.999%, JINGHUI GAS). Deionized water obtained from water purifier (Master-S15 UV) was used throughout all experiments.
1.2 Preparation of CatalystsThe schematic diagram of catalyst synthesis was shown in Scheme 1.
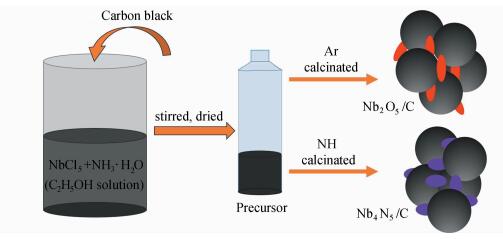
|
Scheme1 Preparation of catalysts Nb2O5/C and Nb4N5/C |
The precursor powders were prepared by a simple wet chemical method. In detail, 0.5 g niobium chloride (NbCl5) was dissolved in 10 mL ethanol and stirred until completely dissolved. Then, 20 mL ammonia (5% aq.) was added to above solution slowly and stirred well to obtain a white emulsion. Within minutes, 1 g of carbon black (C) was added to the white emulsion. After completion of the reaction, the emulsion was heated by oil bath at 100 ℃ and then evaporated to obtain Nb-based precursor.
1.2.2 Synthesis of Nb4N5/ C and Nb2O5/C0.5 g of precursor was placed in a porcelain boat and loaded into a quartz tube in a tube furnace. The tube furnace was placed under a constant 100 mL/min flow of NH3 or Ar and then heated to given temperature (600 ℃, 700 ℃, 800 ℃ in NH3 and 700 ℃ in Ar) for 3 h with a rate of 2 ℃/min before cooling to room temperature.
1.3 Characterizations and AnalysesThe crystalline structures of all the samples were analyzed by an X-ray diffractometer (XRD; D8 focus, Bruker) with CuKα1 radiation (λ=1.54056 Å). Morphology and size of the materials was examined through transmission electron microscopy (TEM; JEM-2100F, JEOL). Element composition and valence state of elements was measured by X-ray photoelectron spectroscopy (XPS; ESCALab250Xi, Thermo Scientific).
The electrochemical measurements were evaluated in a gas-tight three electrode cell. Pt wire was the counter electrode, and Saturated Calomel Reference Electrode (SCE) was the reference electrode. The working electrodes were fabricated through dropping sample suspension onto the pre-polished glass carbon electrode. The sample suspension was prepared as follows: 5 mg of Niobium nitride/carbon was dispersed in 10 μL Nafion (5%, mass fraction) and 500 μL ethanol, and sonicated for 1 h to form homogeneous suspension. Then 5 μL of the well-dispersed catalyst suspension was loaded onto the pre-polished glassy carbon electrode (3 mm in diameter). After drying at room temperature, 2.0 μL Nafion solution (0.05%, mass fraction) was poured onto the surface of the catalyst layer to form a thin protective film and then dried in air. Finally, the as-prepared electrode was used for CO2 electrochemical reduction.
The cyclic voltammetry (CV) and controlled potential electrolysis measurements were recorded in Ar or CO2 saturated 0.5 mol·L-1 NaCl solution. The evolved gases were detected by a gas chromatograph (SRI 8601C MG#1, USA). Liquid phase product was detected by the Nuclear Magnetic Resonance. The potentials in the study were reported versus RHE with the conversion E (vs. RHE)=E (vs. SCE)+0.245 V+0.059×pH.
2 Results and Discussion 2.1 Characterization of CatalystsAs shown in Figure 1, the composition of Nb/C precursor without treatment in NH3 or Ar was HNbO3 (PDF#36-0794), and four strong characteristic peaks at 22.9°, 32.6°, 46.8° and 58.2° correspond to the crystal facets (200), (222), (400) and (422) respectively. Nb4N5/C catalysts were prepared by NH3 treatment of the precursor at different temperature, and named as Nb4N5/C-600, Nb4N5/C-700, Nb4N5/C-800, respectively. XRD patterns of Nb4N5/C catalysts showed that the crystal structure matched with PDF#51-1327, characteristic peaks at 36.1°, 41.7°, 60.9° and 73.2° corresponding to the crystal facets (211), (310), (312) and (213). The crystallinity of Nb4N5 gradually increased as the nitriding temperature increased, suggesting better formation of the materials. The Nb2O5/C catalyst was obtained through pyrolysis in Ar atmosphere at 700 ℃, corresponding to PDF#30-0873, and the characteristic peaks at the angle of 22.6°, 28.4°, 36.6°, 50.9°, and 55.1° was in agreement with crystal facets (001), (180), (181), (380) and (182), respectively.
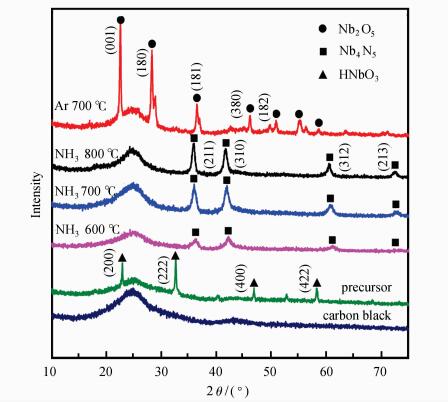
|
Figure 1 XRD patterns of carbon black, precursor, Nb4N5/C-600, Nb4N5/C-700, Nb4N5/C-800 and Nb2O5/C |
X-ray photoelectron spectroscopy (XPS) measurements were used to study the surface chemical composition and oxidation state of the samples. XPS survey spectrum shown in Figure 2(a) showed that catalyst Nb4N5/C-700 was composed of four elements: C, N, O and Nb, and no other elements were found, indicating that no impurities were introduced during the preparation.
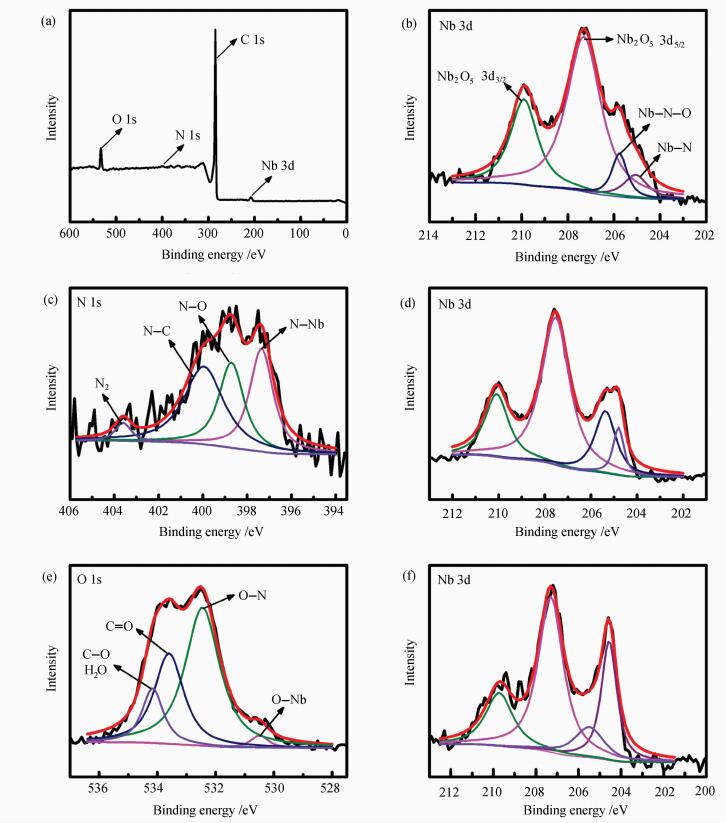
|
Figure 2 (a) survey, (c) N 1s, (e) O 1s of XPS spectra for Nb4N5/C-700 and Nb 3d XPS spectra of (b) Nb4N5/C-600, (d) Nb4N5/C-700, (f) Nb4N5/C-800 |
The N 1s high-resolution spectrum could be fitted with four peaks, as shown in Figure 2(c), at the binding energy of 397.3 eV, 398.7 eV, 400.0 eV and 403.6 eV corresponding to N—Nb, N—O, N—C bonds and N2 adsorbed on the surface, respectively[18]. The O 1s high-resolution spectrum shown in Figure 2(e) was also fitted with four peaks located at 530.5 eV, 532.4 eV, 533.6 eV and 534.1 eV which could be associated with O—Nb, O—N, C=O and C—O[21] bonds or adsorbed water. The Nb 3d high-resolution spectra of the above three catalysts mainly contained three obvious peaks, which was deconvoluted into four sub-peaks. As shown in Figure 2(b), the two binding energy peaks at 209.9 eV and 207.3 eV can be assigned to Nb 3d3/2 and Nb 3d5/2 belonged to Nb2O5[22].It was demonstrated that oxidation has taken place on the surface of the catalyst, commonly reported in previous studies[23].The other two sub-peaks at the binding energy of 205.7 eV and 205.0 eV was identified as Nb—N—O and Nb—N[24], respectively. In addition, from XPS result presented in Figure 2(b), 2(d) and 2(f), with the nitriding temperature increasing, from 600 ℃ to 800 ℃, the area of the peak containing N element (Nb—N—O and Nb—N) increases which was demonstrated that the nitrogen content of the catalysts increased. More information of Nb 3d XPS spectra for catalysts was shown in Table 1.
| Table 1 Nb 3d XPS spectra information of catalysts and N content |
The morphology of Nb4N5/C and Nb2O5/C was characterized by TEM, shown in Figure 3. The Nb4N5 nano-particles were approximately spherical with average diameter of 13 nm. The crystal plane spacing of 0.25 nm corresponds to the crystal facet (211), consistent with the XRD pattern of Nb4N5/C-700 in Figure 1. After calcination in Ar atmosphere, Nb/C precursor whose main component was HNbO3 changed into Nb2O5. As presented in Figure 3(d), the main crystal plane spacing was 0.40 nm corresponding to the crystal facet (001) which was consistent with XRD pattern named Nb2O5/C in Figure 1.
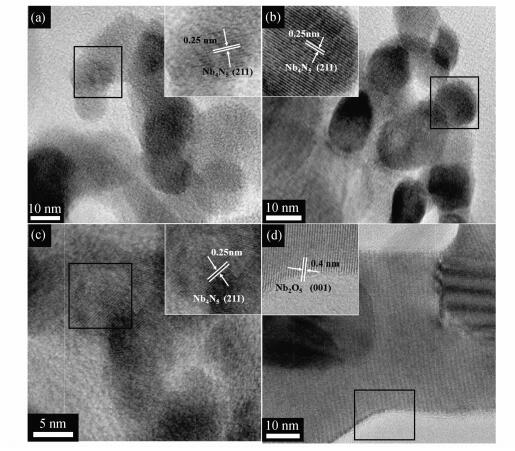
|
Figure 3 High-resolution TEM images of catalysts (a) Nb4N5/C-600, (b) Nb4N5/C-700, (c) Nb4N5/C-800, (d) Nb2O5/C |
In order to investigate the element distribution of the carbon nanoparticles and Nb4N5, the EDS mappings of elemental C, N and Nb were carried out and shown in Figure 4(a). As observed, elements of C distributed uniformly while elements of Nb and N showed a distinct island distribution on carbon substrate. It was demonstrated that carbon powder was decorated by Nb4N5 nanoparticles effectively which was beneficial to the continous electrolysis process, contributing to the excellent catalytic activity. The EDS spectrum of Nb4N5/C-700 shown in Figure 4(b) further verified the high purity of the catalyst (the Cu and O signals in this figure were from the carbon film used to capture the electron microscope) which was consistent with the result of XPS shown in Figure 2(a).

|
Figure 4 (a) Elemental mapping of catalyst Nb4N5/C-700, (b) EDS spectrum of select area |
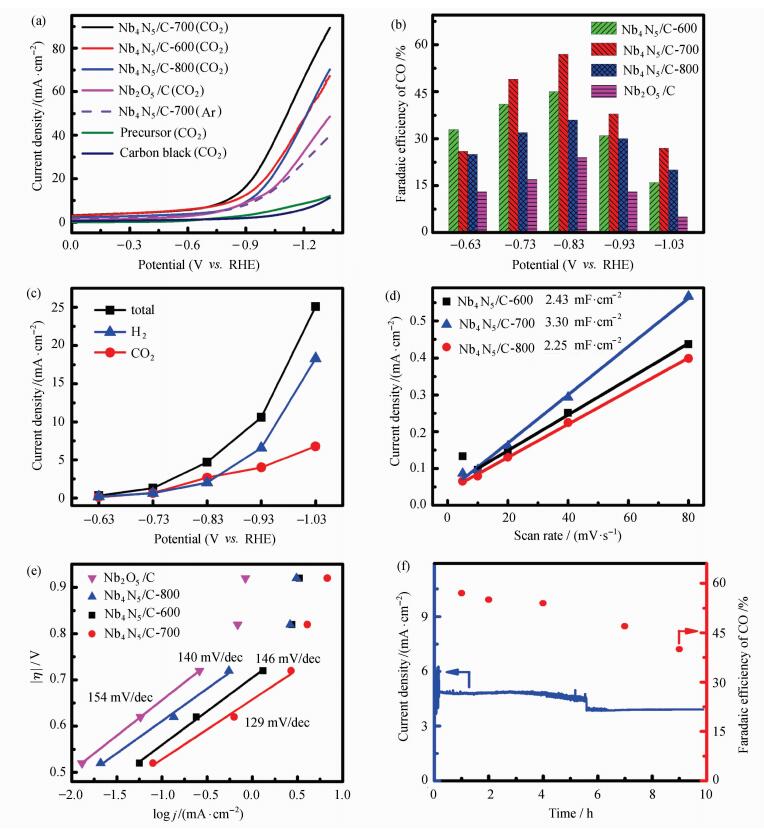
Electrochemical properties of Nb4N5/C were examined. As presented in Figure 5(a), the current density of Nb4N5/C-700 under CO2 was larger than that under Ar, demonstrating that Nb4N5/C-700 had high CO2RR catalytic activity. In contrast with the bare carbon black and precursor, all of the Nb4N5/C catalysts exhibited significantly larger current density, which means higher catalytic activity[25].The electrolysis data also show that Nb4N5/C-700 exhibited higher catalytic activity in CO2 reduction compared to carbon black, precursor and Nb2O5/C.
|
Figure 5 (a) LSV of different samples in CO2 saturated 0.5 mol·L-1 NaCl electrolyte with scan rate at 50 mV·s-1. (b) Faradaic efficiency of CO by Nb4N5/C and Nb2O5/C. (c) Current density for total, CO and H2 of Nb4N5/C-700 at various applied potentials. (d) Charging current density differences plotted against scan rate of catalysts Nb4N5/C- 600, 700, 800. (e) Tafel plots for CO by Nb4N5/C- 600, 700, 800 and Nb2O5/C in CO2 saturated 0.5 mol·L-1 NaCl electrolyte. (f) Current density and Faradic efficiency of CO during 10 hours at -0.83 V by Nb4N5/C-700 |
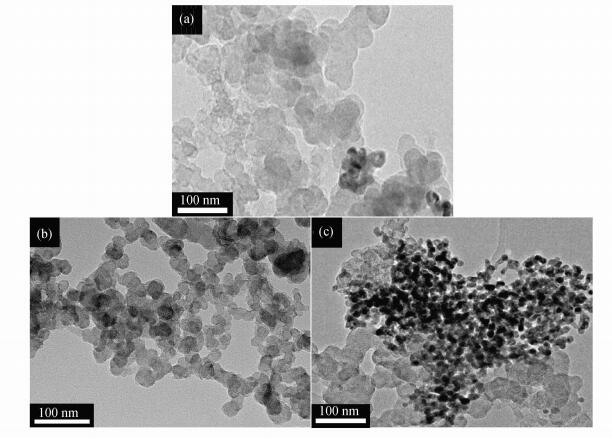
CO2 reduction electrocatalysis was performed on Nb4N5/C and Nb2O5/C. H2 and CO were the only products in CO2RR and no liquid products were detected. Figure 5(b) showed that Nb4N5/C catalysts exhibited high catalytic activity. Nb4N5/C-700 showed the highest catalytic activity among these catalysts. With increased potential, the Faradaic efficiency of CO was increasing and reached to the maximum of 57% at -0.83 V, higher than that of Nb4N5/C-600 (FEco=45%) and Nb4N5/C-800 (FEco=36%). The partial current density of CO was also increasing from 0.08 mA·cm-2 to 6.78 mA·cm-2 with the applied potential increasing, as presented in Figure 5(c). As observed, with the nitriding temperature increasing, the N doping of Nb4N5 became higher (Figure 2), and formed more reaction sites for CO2RR. However, when the temperature was too high, the nanoparticles of Nb4N5 agglomerated, as seen in Figure 6, and the electrochemically active surface area (EACS) of catalyst and the number of active sites was reduced, therefore the catalytic activity decreased. The double-layer capacitance (Cdl), proportional to the EACS, was calculated using the relationship between current and scan rate in non-Faradaic region. As shown in Figure 5(d), the Cdl value of catalysts Nb4N5/C-600, Nb4N5/C-700 and Nb4N5/C-800 is 2.43 mF·cm-2, 3.30 mF·cm-2 and 2.25 mF·cm-2 respectively. The higher value, due to high N doping and small size of the catalyst, means more active sites in catalyst Nb4N5/C-700. In addition, the Cdl value of Nb4N5/C-800 (2.25 mF·cm-2) was smaller than that of Nb4N5/C-700 (3.30 mF·cm-2), which illustrated that the agglomeration of the catalyst reduces its specific surface area which was consistent with Figure 6.
|
Figure 6 TEM images of (a) Nb4N5/C-600, (b) Nb4N5/C-700 and (c) Nb4N5/C-800 |
Tafel slope shows the inherent property of electrocatalysis, which is determined by a rate-limiting step. As shown in Figure 5(e), Nb4N5/C-700 had a Tafel slope of 129 mV/dec, smaller than 154 mV/dec, 140 mV/dec and 146 mV/dec for Nb2O5/C, Nb4N5/C-600 and Nb4N5/C-800, respectively. This result indicated that Nb4N5/C-700 had good intrinsic performance in CO2RR and exhibited much faster electron transfer compared with the others. In addition, the slope is close to 118 mV/dec, demonstrating that the single electron transfer process (CO2+e-CO2·-) is the rate determine step of the reaction[2]. Long term electrolysis measurement, as presented in Figure 5(f), indicated that the current density of the system was still maintained at more than 70% of the initial state, and the Faradaic efficiency of CO slightly decreased after 6 hours, but remained above 40%. This result illustrated that catalysts Nb4N5/C-700 has relatively good stability.
3 ConclusionsIn summary, Nb4N5/C were prepared by a simple wet chemical method and calcination at NH3 atmosphere, which increased the performance in CO2RR. Nb4N5/C calcinated at different nitriding temperatures 600 ℃, 700 ℃ and 800 ℃ all had catalytic ability of CO2RR. Nb4N5/C-700 exhibited good catalyst activity with Faradaic efficiency of CO reaching 57% at -0.83 V vs RHE in CO2 saturated 0.5 mol·L-1 NaCl solution. The results showed that the catalytic activity of the catalyst was related to the N doping and the degree of agglomeration of Nb4N5. The N doping can provide more active sites for CO2 reduction, and increases at higher temperature. However, agglomeration of the catalyst also increased above 700 ℃. Thus, Nb4N5/C-700 was more balanced with suitable N doping and without excessive agglomeration, showing the best activity. In conclusion, increasing the degree of N doping and reducing the agglomeration of the nanoparticles were beneficial to the electrocatalytic reduction of CO2. This work also provides a new method of increasing catalytic efficiency.
| [1] |
Chu S, Majumdar A. Opportunities and challenges for a sustainable energy future[J]. Nature, 2012, 488(7411): 294-303. DOI:10.1038/nature11475 |
| [2] |
Zheng T, Jiang K, Wang H. Recent advances in electrochemical CO2-to-CO conversion on heterogeneous catalysts[J]. Advanced Materiaks, 2018, e1802066. |
| [3] |
Centi G, Quadrelli E A, Perathoner S. Catalysis for CO2 conversion:a key technology for rapid introduction of renewable energy in the value chain of chemical industries[J]. Energy & Environmental Science, 2013, 6(6): 1711-1731. |
| [4] |
Lee S Y, Jung H, Kim N K, Oh H S, Min B K, Hwang Y J. Mixed copper states in anodized Cu electrocatalyst for stable and selective ethylene production from CO2 reduction[J]. Journal of the American Chemical Society, 2018, 140(28): 8681-8689. DOI:10.1021/jacs.8b02173 |
| [5] |
Liu M, Pang Y, Zhang B, De Luna P, Voznyy O, Xu J, Zheng X, Dinh C T, Fan F, Cao C, de Arquer F P, Safaei T S, Mepham A, Klinkova A, Kumacheva E, Filleter T, Sinton D, Kelley S O, Sargent E H. Enhanced electrocatalytic CO2 reduction via field-induced reagent concentration[J]. Nature, 2016, 537(7620): 382-386. DOI:10.1038/nature19060 |
| [6] |
Lu Q, Rosen J, Zhou Y, Hutchings G S, Kimmel Y C, Chen J G G, Jiao F. A selective and efficient electrocatalyst for carbon dioxide reduction[J]. Nature Communications, 2014, 5: 6. |
| [7] |
Sekar P, Calvillo L, Tubaro C, Baron M, Pokle A, Carraro F, Martucci A, Agnoli S. Cobalt spinel nanocubes on N-doped graphene:a synergistic hybrid electrocatalyst for the highly selective reduction of carbon dioxide to formic acid[J]. ACS Catalysis, 2017, 7(11): 7695-7703. DOI:10.1021/acscatal.7b02166 |
| [8] |
Schreier M, Luo J, Gao P, Moehl T, Mayer M T, Gratzel M. Covalent immobilization of a molecular catalyst on Cu2O photocathodes for CO2 reduction[J]. Journal of the American Chemical Society, 2016, 138(6): 1938-1946. DOI:10.1021/jacs.5b12157 |
| [9] |
Ren D, Deng Y, Handoko A D, Chen C S, Malkhandi S, Yeo B S. Selective electrochemical reduction of carbon dioxide to ethylene and ethanol on Copper(Ⅰ) oxide catalysts[J]. ACS Catalysis, 2015, 5(5): 2814-2821. DOI:10.1021/cs502128q |
| [10] |
Geng Z G, Kong X D, Chen W W, Su H Y, Liu Y, Cai F, Wang G X, Zeng J. Oxygen vacancies in ZnO nanosheets enhance CO2 electrochemical reduction to CO[J]. Angewandte Chemie-International Edition, 2018, 57(21): 6054-6059. DOI:10.1002/anie.201711255 |
| [11] |
Gu J, Heroguel F, Luterbacher J, Hu X. Densely packed, ultra small SnO nanoparticles for enhanced activity and selectivity in electrochemical CO2 reduction[J]. Angewandte Chemie International Ed. in English, 2018, 57(11): 2943-2947. DOI:10.1002/anie.201713003 |
| [12] |
Zhu D D, Liu J L, Qiao S Z. Recent advances in inorganic heterogeneous electrocatalysts for reduction of carbon dioxide[J]. Advanced Materials, 2016, 28(18): 3423-3452. DOI:10.1002/adma.201504766 |
| [13] |
Schlogl R. Heterogeneous catalysis[J]. Angewandte Chemie. International Ed. in English, 2015, 54(11): 3465-3520. DOI:10.1002/anie.201410738 |
| [14] |
Yang J, Hao J Y, Xu S Y, Dai J, Wang Y, Pang X C. Visible-light-driven photocatalytic degradation of 4-CP and the synergistic reduction of Cr(Ⅵ) on one-pot synthesized amorphous Nb2O5 nanorods/graphene heterostructured composites[J]. Chemical Engineering Journal, 2018, 353: 100-114. DOI:10.1016/j.cej.2018.07.115 |
| [15] |
de Moraes N P, da Silva M, Rodrigues L A. Effect of metal doping in the photocatalytic properties of carbon xerogel-Nb2O5 composite towards visible light degradation of methylene blue[J]. Materials Letters, 2018, 228: 486-489. DOI:10.1016/j.matlet.2018.06.095 |
| [16] |
Shen H, Wei B B, Zhang D F, Qi Z B, Wang Z C. Magnetron sputtered NbN thin film electrodes for supercapacitors[J]. Materials Letters, 2018, 229: 17-20. DOI:10.1016/j.matlet.2018.06.052 |
| [17] |
da Silva G T S T, Carvalho K T G, Lopes O F, Ribeiro C. g-C3N4/Nb2O5 heterostructures tailored by sonochemical synthesis:enhanced photocatalytic performance in oxidation of emerging pollutants driven by visible radiation[J]. Applied Catalysis B:Environmental, 2017, 216: 70-79. DOI:10.1016/j.apcatb.2017.05.038 |
| [18] |
Cui H, Zhu G, Liu X, Liu F, Xie Y, Yang C, Lin T, Gu H, Huang F. Niobium nitride Nb4N5 as a new high-performance electrode material for supercapacitors[J]. Advanced Science, 2015, 2(12): 1500126. DOI:10.1002/advs.201500126 |
| [19] |
Dong C, Wang X, Liu X, Yuan X, Dong W, Cui H, Duan Y, Huang F. In situ grown Nb4N5 nanocrystal on nitrogen-doped graphene as a novel anode for lithium ion battery[J]. RSC Advances, 2016, 6(84): 81290-81295. DOI:10.1039/C6RA13647H |
| [20] |
Zhang M, Hou P, Wang Z, Kang P. Nitrogen-doped Ta2O5 nanocomposites for the electrocatalytic reduction of carbon dioxide to CO with photoassistance[J]. ChemElectroChem, 2018, 5(5): 799-804. |
| [21] |
Thiede T B, Parala H, Reuter K, Passing G, Kirchmeyer S, Hinz J, Lemberger M, Bauer A J, Barreca D, Gasparotto A, Fischer R A. Deposition of niobium nitride thin films from tert-butylamido-tris-(diethylamido)-niobium by a modified industrial MOCVD reactor[J]. Chemical Vapor Deposition, 2009, 15(10-12): 334-341. |
| [22] |
Alfonso J E, Buitrago J, Torres J, Marco J F, Santos B. Influence of fabrication parameters on crystallization, microstructure, and surface composition of NbN thin films deposited by rf magnetron sputtering[J]. Journal of Materials Science, 2010, 45(20): 5528-5533. DOI:10.1007/s10853-010-4612-3 |
| [23] |
Jouve G, Severac C, Cantacuzene S. XPS study of NbN and (NbTi)N superconducting coatings[J]. Thin Solid Films, 1996, 287(1-2): 146-153. DOI:10.1016/S0040-6090(96)08776-7 |
| [24] |
Ufuktepe Y, Farha A H, Kimura S I, Hajiri T, Imura K, Mamun M A, Karadag F, Elmustafa A A, Elsayed-Ali H E. Superconducting niobium nitride thin films by reactive pulsed laser deposition[J]. Thin Solid Films, 2013, 545: 601-607. DOI:10.1016/j.tsf.2013.08.051 |
| [25] |
Shearer C J, Cherevan A, Eder D. Application and future challenges of functional nanocarbon hybrids[J]. Advanced Materials, 2014, 26(15): 2295-2318. DOI:10.1002/adma.v26.15 |




