Dye-sensitized solar cells (DSCs) reported in 1991 are regarded as low-cost next generation solar cells, and significant progress has been made in the past few decades[1-3]. In DSCs, the sensitizers play a crucial role due to their critical function in light harvesting and electron injection[4]. Uptill now, DSCs based on metal-complex sensitizers such as Ru complexes and Zn porphyrin photosensitizers have achieved efficiencies up to 11% and 13%, respectively[5, 6]. However, the limitations corresponding to these complexes such as the rare resources of ruthenium and zinc metals, high manufacturing cost, and environmental issues would restrain their widespread applications[7]. So other organic metal-free sensitizers have attracted much attention for researchers in view of their advantages, such as larger molar extinction coefficients, higher structural flexibility, lower cost and environmentally friendliness[8, 9]. It was worthy noting that the record highest efficiency of 12.5% has been achieved by Wang and coworkers[10].
Generally, metal-free organic sensitizers are constituted by donor-(π conjugated spacer)-acceptor (D-π-A) structure. However, the absorption spectra of most of the D-π-A dyes are narrow, which is not beneficial for higher short-circuit current (Jsc). Therefore, further studies are needed to develop to reduce the charge recombination and broaden the range of light-harvesting. Co-sensitization is an effective approach, by which a combination of two or more dyes adsorbed together on semiconductor surface, extending the light-harvesting ability to enhance the short-circuit photocurrent density (Jsc) and thus the efficiency (η) of DSCs[11]. Ogura et al. used a co-sensitizer composing terpyridine complex (black dye) with an indoline dye (D131) and resulted in a significantly enhanced photocurrent, leading to a device performance of 11%[12]. Ko et al. explored a Ru complex (JK-142) as sensitizer in combination with an organic dye (JK-62), and positioned them on TiO2 film[13]. Surprisingly a considerably improved efficiency of up to 10.2% was achieved, which is favorably superior to that of N719 (8.68%) in the same device configurations. In 2011, a DSC with a high photovoltaic performance of 12.3% was demonstrated with the co-sensitization of a zinc porphyrin dye YD2-o-C8 and a metal-free organic dye Y123 with cobalt (Ⅱ/Ⅲ)-based redox electrolyte[14]. Of course, there are also successful examples of co-sensitized DSCs using solely metal-free organic dyes. For instance, Choi et al. achieved a high performance of 8.65% by co-sensitizing JK2 and SQ1 on Al2O3-coated TiO2 films[15]. Sun et al. applied cyanine dye CM203 to co-sensitize with other two organic dyes (CMR103 and HY113), the Jsc was markedly increased due to the spectra response to the whole visible domain, and consequently the conversion efficiency of 8.2% was achieved[16]. In addition to much emphasis on the effect of spectral complementation ameliorating the Jsc mentioned in the above research, dyes in co-sensitized system also have some influence on open circuit voltage (Voc) and then Jsc or η. Almost all the Jsc and Voc of co-sensitized cells exhibit different degree of increase compared with those of DSCs based on individual dyes.
As a part of our ongoing efforts to design and synthesize triphenylamine-based organic dyes, we also interested in co-sensitizing multiple dyes for improving the performance of DSCs[17-20]. We noticed an interesting trend in which sensitizers incorporating cyanoacrylic acid (CA) moiety as the acceptor/anchoring group commonly exhibited higher Voc values than the corresponding ones bearing identical donors and π-bridges but a different acceptor of rhodanine-3-acetic acid (RA) moiety. In the present work, the co-sensitization of two metal-free triphenylamine dyes with different acceptor moieties (RA and CA) is applied to DSCs. Furthermore, the relationship between the photovoltaic properties and triphenylamine dyes are also discussed in detail.
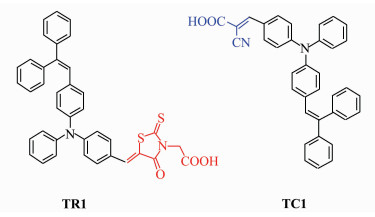
2 Experiment 2.1 Preparation of Dye-sensitized Nanocrystalline TiO2 Electrodes and CharacterizationThe two triphenylamine dyes TR1 and TC1 with different acceptor moieties were synthesized following the previous reports[20, 21]. The chemical structures of these dyes are shown in Figure 1. Nanocrystalline TiO2 films were prepared by coating TiO2 paste onto the F-doped transparent conductive glass (F-doped SnO2, short for FTO) as described by Grätzel and coworkers[22]. Briefly, FTO substrates (10 Ω/sq, >85% transparency in the visible region, Yaohua, China) were treated with TiCl4 (aq., 50 mmol/L) for 30 min, followed by screen-printing a paste consisted of TiO2 (P25, a mixture of 30% rutile and 70% anatase, Degussa AG, Germany; 16%), ethyl cellulose (20-30 cp, 5%) and terpinol (79%). The film was successively fired at 450 ℃ under air for 30 min, treated with TiCl4 solution and fired again to give a ~15 μm thick mesoscopic TiO2 film.
|
Figure 1 Molecular structures of triphenylamine dyes TR1 and TC1 |
Dyes TR1 and TC1 were dissolved individually in absolute methanol to form dye-sensitizer solutions with equal concentration (5×10-5 mol/L) and volume (10 mL). Mixing TR1 and TC1 solutions with different volume ratios of 9:1, 7:3, 5:5, 3:7 and 1:9 would get the co-sensitizer solutions, in which the total concentration and volume are still the same as those of individual dye solutions. Dye sensitization was carried out by immersing the TiO2 electrodes in TR1 or TC1 solutions while the TiO2 films were still hot. This process gave TR1/TiO2 and TC1/TiO2 electrodes. Co-sensitized electrodes were obtained by immersing TiO2 films in the prepared mixed solutions with TR1 to TC1 molar ratios of 9:1, 7:3, 5:5, 3:7 and 1:9, which were coded as Co1/TiO2, Co2/TiO2, Co3/TiO2, Co4/TiO2 and Co5/TiO2, respectively. The absorption spectra of the dyes in methanol solutions and adsorbed on TiO2 films were measured with a Jasco V-550 UV/Vis spectrophotometer.
2.2 Fabrication and Characterization of Dye-sensitized Solar CellsThe dye-sensitized TiO2 photoanode and the platinum coated FTO photocathode were separated by adhesive tapes (45 μm thick) to form a space, in which a redox electrolyte (0.1 mol/L LiI, 0.05 mol/L I2, and 0.6 mol/L DMPImI in acetonitrile) be introduced. The cells were finalized by firmly clamping the two electrodes together.
For photovoltaic measurements of the DSCs, a 500 W Xe lamp (400-800 nm) served as the light source in combination with a optical filter to remove ultraviolet and infrared radiation. The light intensity was held at 100 mW/cm2 at the surface of the test cells with the aid of a radiant power meter (Oriel Model 70260 with 70268 Probe). I-V characteristics were recorded with a digital source meter (Keithley 2400) controlled by a computer. Monochromatic incident photon to current conversion efficiency (IPCE), plotted as a function of excitation wavelength, was recorded on a home-built system equipped with a bromine tungsten lamp and a grating spectrometer (SBP300, Zolix). The IPCE system was calibrated using a silicon reference cell (the 18th Research Institute of Electronics Industry Ministry, China). Electrochemical impedance spectra (EIS) measurements of the DSCs were carried out using a potentiostat/galvanostat/FRA (PARSTAT 2273) in the dark with the frequency range of 100 mHz to 100 kHz. The applied bias voltage and alternate current amplitude were set at -0.65 V and 0.01 V, respectively. The resulting curves were fitted to the appropriate equivalent circuit using Z-view software.
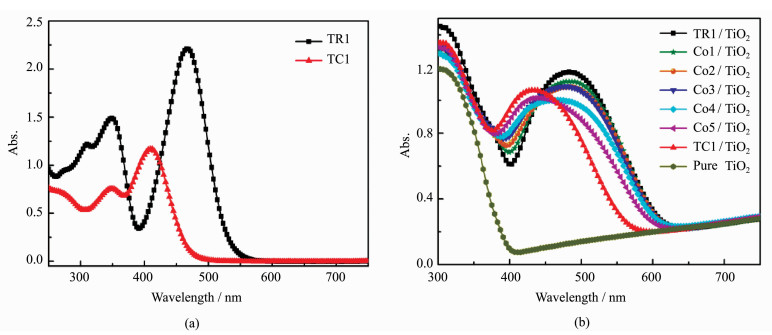
3 Results and DiscussionsThe absorption spectra of dyes TR1 and TC1 in absolute methanol solution are shown in Figure 2(a). The absorption peaks of TR1 and TC1 in methanol are at 470 and 410 nm. The corresponding maximum molar extinction coefficients (εmax) are 4.47×104 mol-1·L·cm-1 and 2.35×104 mol-1·L·cm-1, which are higher than those of Ru complexes (~1.4 × 103 mol-1·L·cm-1), indicating a good ability of light harvesting[23]. Noticeably, the photoresponse could complement to each other to some extent, especially in the range of 380-550 nm.
|
Figure 2 Absorption spectra of dyes TR1 and TC1 in methanol solution (a), and all TiO2 films sensitized in different sensitizer solutions with different volume ratios, with that of pure TiO2 film as a reference (b) |
Figure 2(b) shows the absorption spectra of TiO2 films sensitized by dyes TR1 and TC1, with that of pure TiO2 film as a reference. In comparison to the spectra of dyes in methanol solution, the absorption spectra on TiO2 films are broadened and red-shifted from 470 nm to 486 nm for TR1, and 410 nm to 431 nm for TC1, implying that the formation of dye aggregates on TiO2 films[24]. Spectra of TiO2 films sensitized by co-sensitizer solutions are also shown in Figure 2(b). In general, the absorption intensity ratio of dye TR1 part to TC1 part became smaller with the concentration increase of dye TC1, but with the absorption onset at almost the same position.
According to previous report[20], the oxidation potential (Eox) of dyes TR1 and TC1 are 1.15 V and 1.18 V vs. normal hydrogen electrode (NHE), while the excited state reduction potentials (Ered) calculated from Eox and the 0-0 energy (E0-0) are -1.19 V and -1.37 V vs. NHE, respectively. These results confirm that electron injection to the conduction band of TiO2 (Ecb=-0.5 V vs. NHE) and regeneration of oxidized dyes by accepting electrons from I- ions (0.4 V vs. NHE for I3-/I- redox couple) are thermodynamically favorable[25]. Although the excited state energy would be changed after the two dyes TR1 and TC1 adsorbing on TiO2 films due to dye aggregation, this change is still relatively small about 0.05-0.1 V[26]. So, this change does not affect the electron injection. In addition, it worth noting that dye TC1 has higher driving force than dye TR1, which would be benefit for cell efficiency improvement when co-sensitized TR1 with TC1.
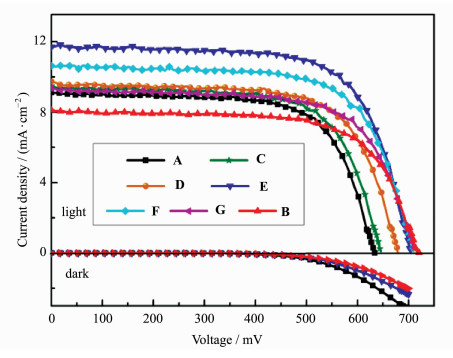
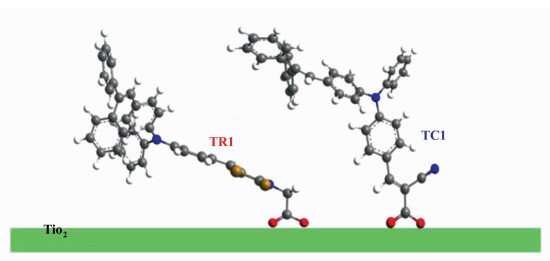
Figure 3 shows the current-voltage characteristics of DSCs sensitized by individual dyes TR1 and TC1 (Devices A, B). The corresponding photovoltaic parameters (Jsc, Voc, FF and η) of these DSCs are summarized in Table 1. Device B shows significant higher Voc value (720 mV) than that of Device A (633 mV), but lower Jsc value (8.10 mA/cm2) than TR1 (9.10 mA/cm2). Many research groups, including ours, have attributed the higher Voc values of TC dyes to their adsorption mode on TiO2[20]. TC dyes with standing adsorption mode have larger adsorption angles, and show larger dipole moments in the direction normal to the TiO2 surface than TR dyes (lie down along the TiO2 surface) (Figure 4). It is likely to shift the conduction band of TiO2 to more negative position and generate higher Voc values[20]. Additionally, the charge recombination is relatively reduced for Device B, which is in consistent with the decreased order of dark current for comparing TC1-based cell device to TR1-based one (Figure 3), and thus benefit for voltage improvement. Finally, efficiencies of 4.15% and 4.01% are obtained for Devices A and B, respectively. And dye TR1 is subjected to co-sensitize with dye TC1 because the latter could compensate for the deficit of the former in the photoresponse and charge recombination.
|
Figure 3 Current density-voltage characteristics of DSCs (Devices A-G) sensitized by TR1, TC1 and co-sensitizers; and dark-current of Devices A, B and E |
| Table 1 The photovoltaic parameters of DSCs sensitized by different organic dyes |
|
Figure 4 Adsorption mode of the two triphenylamine dyes on TiO2 surface |
DSCs sensitized by dye mixture solutions (Devices C-G) with different molar ratios are also characterized. It can be seen from Figure 3 and Table 1 that, Voc are increasing all along with the increasing adsorbed amount of dye TC1 obviously. Because dye TC1 molecules occupy some positions of dye TR1, the charge recombination would be reduced for reasons mentioned above. For example, the dark current of Device E is decreased than Device A, but serious than Device B (Figure 3). And the more the TC dye, the smaller the dark current is. On the other hand, Jsc values are not always increasing by decreasing the co-sensitizer molar ratio of the mixture solutions (from Device C to Device G). It is intriguing that the largest Jsc value reaches 11.7 mA/cm2 for Device E. Then the Jsc and η became to decrease, but still higher than solar cells sensitized by individual dyes. The highest efficiency of 6.03% is achieved for Device E sensitized by the mixture solution containing equivalent concentration. These results indicate that co-sensitization of the two dyes is an effective strategy to improve the efficiency of DSCs.
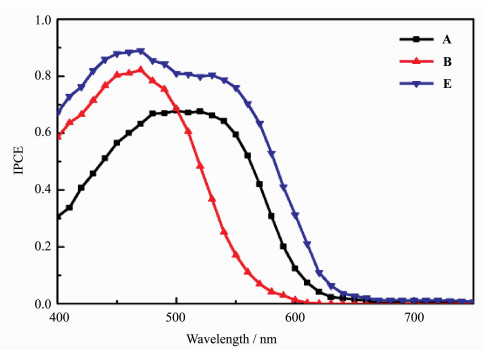
Figure 5 presents the IPCE spectra of solar cells using the individual dyes (Devices A and B). Although the photoresponse range of TC1 dye is narrower than the corresponding TR1 dye, a rather higher IPCE plateau value for TC1-sensitized solar cell (Device B) is performed. It is probably due to better electron injection into TiO2 for TC1 dye and/or the reduced charge recombination that make the charge collection efficiency increase at the photoanode. The corresponding IPCE spectrum of cell Device E is displayed also in Figure 5. It is found to be broadened by covering the visible domain from 400 to 700 nm in comparison with those of solar cells sensitized by individual dyes TR1 and TC1. The peak values of 80% and 88% are at 530 and 460 nm, respectively, corresponding to the absorption spectra of dye mixtures on TiO2 film.
|
Figure 5 IPCE spectra of Devices A, B and E |
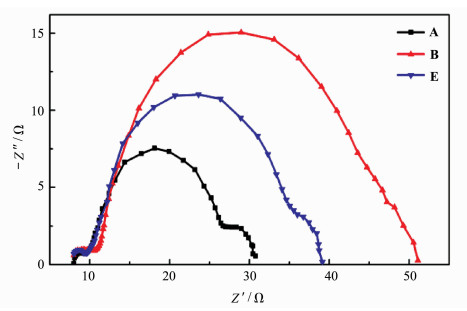
Because of the great importance of electron transport properties on the performance of DSCs, it is investigated for the Devices A, B and E based on different sensitizing system with EIS technique[27-30]. Figure 6 shows the impedance data of solar cells A, B and E measured in the dark under -0.65 V applied bias. Three typical semicircles in the Nyquist plots were observed, and the middle-frequency semicircles correspond to electron recombination at the TiO2/electrolyte interface together with electron transport in the TiO2 network. Fitting these middle-frequency semicircles subsequently gives the charge-transfer resistance (Rct), and the chemical capacitance (Cμ) at the TiO2/electrolyte interface[31-33]. The fitted Rct values vary from one device to another in the sequence of Device B (31 Ω) > Device E (24 Ω) > Device A (17 Ω), which corresponds to the order of dark current shown in Figure 3. It indicated that recombination of conduction band electrons with the electrolyte occurs more easily in a reverse order. The Cμ values decrease with the order of Device B (679 μF) > Device A (534 μF) > Device E (501 μF). By these fitted Rct and Cμ results, electron lifetime τr according to the equation τr =Cμ×Rct could be obtained. The τr value of Device E based on co-sensitizer is 11.6 ms, which is in between the DSCs based on the two individual dyes, i.e. for Device A (8.8 ms) and Device B (20.4 ms). This trend is in good accordance with that for Voc. The longer electron lifetime for DSC based on co-sensitization than Device A may be due to a more compact coverage of dye on TiO2 surface after co-sensitization that block the approach of I3- to the TiO2 surface and decrease the recombination of injected electrons with I3- ions. The increased τr is expected to be the reason for high Jsc, Voc and η of Device E.
|
Figure 6 Impedance spectra of DSCs (Devices A, B and E) measured at -0.65 V bias in the dark |
In conclusion, we demonstrated that co-sensitization by two metal-free triphenylamine dyes with different acceptor groups (TR1 and TC1) is a successful approach to improve the performance of DSCs. The Voc of the cells were increasing all along with the decreased volume ratio (also molar ratio) of TR1:TC1 in the cosensitized solution, resulting from the standing adsorbed TC1 dye molecules take up some positions of TR1 with lied down adsorption mode. While the Jsc of the cells reached the max. at 5:5 molar ratio, which profited from the complimentary spectra response of the two dyes on the one hand, and the enhanced the electron collection efficiency for the decreased dark current on the other hand. The best efficiency of 6.03% was obtained for the cell sensitized by the co-sensitizers with 5:5 molar ratio in mixed solution. These results shown here not only provide new vision on how to produce highly efficient solar cells using dyes with various molecular structures, but also motivate other groups to explore more efficient co-sensitizers through controlling the molecule structure to further enhance the performance of DSCs.
Acknowledgements This work was financially supported by the National Natural Science Foundation of China (21603053), and Natural Science Foundation of Hebei Province (B2014208062, B2014208066).| [1] |
O'Regan B, Grätzel M. Low-cost high-efficiency solar cell based on dye-sensitized colloidal TiO2 films[J]. Nature, 1991, 353: 737-740. DOI:10.1038/353737a0 |
| [2] |
Huang T, Wei H X, Shen J H, Liu Y J, Cheng H Y, Ji Z L, Zhao X. Synthesis and photoelectric properties of dye sensitizers based on phenothiazine as donor[J]. Imaging Science and Photochemistry, 2018, 36(1): 73-81. |
| [3] |
Mehmood U, Ahmad S H A, Khan A U H, Qaiser A A. Co-sensitization of graphene/TiO2 nanocomposite thin films with ruthenizer and metal free organic photosensitizers for improving the power conversion efficiency of dye-sensitized solar cells (DSSCs)[J]. Solar Energy, 2018, 170: 47-55. DOI:10.1016/j.solener.2018.05.051 |
| [4] |
Luo J S, Wan Z Q, Jia C Y, Wang Y, Wu X C, Yao X J. Co-sensitization of dithiafulvenyl-phenothiazine based organic dyes with N719 for efficient dye-sensitized solar cells[J]. Electrochimica Acta, 2016, 211: 364-374. DOI:10.1016/j.electacta.2016.05.175 |
| [5] |
Sauvage F, Decoppet J D, Zhang M, Zakeeruddin S M, Comte P, Nazeeruddin M, Wang P, Grätzel M. Effect of sensitizer adsorption temperature on the performance of dye-sensitized solar cells[J]. Journal of the American Chemical Society, 2011, 133(24): 9304-9310. DOI:10.1021/ja110541t |
| [6] |
Mathew S, Yella A, Gao P, Humphry-Baker R, Curchod B F E, Ashari-Astani N, Tavernelli I, Rothlisberger U, Nazeeruddin M K, Grätzel M. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers[J]. Nature Chemistry, 2014, 6: 242-247. DOI:10.1038/nchem.1861 |
| [7] |
Mishra A, Fischer M K R, Bäuerle P. Metal-free organic dyes for dye-sensitized solar cells: from structure: property relationships to design rules[J]. Angewandte Chemie International Edition, 2009, 48(14): 2474-2499. DOI:10.1002/anie.200804709 |
| [8] |
Urbani M, Ragoussi M E, Nazeeruddin M K, Torres T. Phthalocyanines for dye-sensitized solar cells[J]. Coordination Chemistry Reviews, 2019, 381: 1-64. DOI:10.1016/j.ccr.2018.10.007 |
| [9] |
Rajkumar S, Kumar M N, Suguna K, Muthulakshmi S, Kumar R A. Enhanced performance of dye-sensitized solar cells using natural cocktail dye as sensitizer[J]. Optik-International Journal for Light and Electron Optics, 2019, 178: 224-230. DOI:10.1016/j.ijleo.2018.10.004 |
| [10] |
Yao Z, Zhang M, Wu H, Yang L, Li R Z, Wang P. Donor/acceptor indenoperylene dye for highly efficient organic dye-sensitized solar cells[J]. Journal of the American Chemical Society, 2015, 137(11): 3799-3802. DOI:10.1021/jacs.5b01537 |
| [11] |
Kumara N T R N, Ekanayake P, Lim A, Liew L Y C, Iskandar M, Ming L C, Senadeera G K R. Layered co-sensitization for enhancement of conversion efficiency of natural dye sensitized solar cells[J]. Journal of Alloys and Compounds, 2013, 581: 186-191. DOI:10.1016/j.jallcom.2013.07.039 |
| [12] |
Ogura R Y, Nakane S, Morooka M, Orihashi M, Suzuki Y, Noda K. High-performance dye-sensitized solar cell with a multiple dye system[J]. Applied Physics Letters, 2009, 94: 073308. DOI:10.1063/1.3086891 |
| [13] |
Fan S Q, Kim C, Fang B, Liao K X, Yang G J, Li C J, Kim J J, Ko J. Improved efficiency of over 10% in dye-sensitized solar cells with a ruthenium complex and an organic dye heterogeneously positioning on a single TiO2 electrode[J]. The Journal of Physical Chemistry C, 2011, 115(15): 7747-7754. DOI:10.1021/jp200700e |
| [14] |
Yella A, Lee H W, Tsao H N, Yi C, Chandiran A K, Nazeeruddin M K, Diau E W G, Yeh C Y, Zakeeruddin S M, Grätzel M. Porphyrin-sensitized solar cells with cobalt (Ⅱ/Ⅲ)-based redox electrolyte exceed 12 percent efficiency[J]. Science, 2011, 334: 629-634. DOI:10.1126/science.1209688 |
| [15] |
Choi H, Kim S, Kang S O, Ko J, Kang M S, Clifford J N, Forneli A, Palomares E, Nazeeruddin M K, Grätzel M. Stepwise cosensitization of nanocrystalline TiO2 films utilizing Al2O3 layers in dye-sensitized solar cells[J]. Angewandte Chemie International Edition, 2008, 47(43): 8259-8263. DOI:10.1002/anie.200802852 |
| [16] |
Cheng M, Yang X C, Li J J, Zhang F G, Sun L C. Co-sensitization of organic dyes for efficient dye-sensitized solar cells[J]. ChemSusChem, 2013, 6(1): 70-77. DOI:10.1002/cssc.201200655 |
| [17] |
Liang M, Xu W, Cai F S, Chen P Q, Peng B, Chen J, Li Z M. New triphenylamine-based organic dyes for efficient dye-sensitized solar cells[J]. The Journal of Physical Chemistry C, 2007, 111(11): 4465-4472. DOI:10.1021/jp067930a |
| [18] |
Xu W, Peng B, Chen J, Liang M, Cai F S. New triphenylamine-based dyes for dye-sensitized solar cells[J]. The Journal of Physical Chemistry C, 2008, 112(3): 874-880. DOI:10.1021/jp076992d |
| [19] |
Xu W, Pei J, Shi J F, Peng S J, Chen J. Influence of acceptor moiety in triphenylamine-based dyes on the properties of dye-sensitized solar cells[J]. Journal of Power Source, 2008, 183(2): 792-798. DOI:10.1016/j.jpowsour.2008.05.025 |
| [20] |
Liang Y Y, Peng B, Chen J. Correlating dye adsorption behavior with the open-circuit voltage of triphenylamine-based dye-sensitized solar cells[J]. The Journal of Physical Chemistry C, 2010, 114(24): 10992-10998. DOI:10.1021/jp1023873 |
| [21] |
Pei J, Peng S J, Shi J F, Liang Y L, Tao Z L, Liang J, Chen J. Triphenylamine-based organic dye containing the diphenylvinyl and rhodanine-3-acetic acid moieties for efficient dye-sensitized solar cells[J]. Journal of Power Source, 2009, 187(2): 620-626. |
| [22] |
Ito S, Murakami T N, Comte P, Liska P, Grätzel C, Nazeeruddin M K, Grätzel M. Fabrication of thin film dye sensitized solar cells with solar to electric power conversion efficiency over 10%[J]. Thin Solid Films, 2008, 516(14): 4613-4619. DOI:10.1016/j.tsf.2007.05.090 |
| [23] |
Li H Y, Yang L Y, Tang R L, Hou Y Q, Yang Y Z, Wang H, Han H W, Qin J G, Li Q Q, Li Z. Organic dyes incorporating N-functionalized pyrrole as conjugated bridge for dye-sensitized solar cells: Convenient synthesis, additional withdrawing group on the π-bridge and the suppressed aggregation[J]. Dyes and Pigments, 2013, 99(3): 863-870. DOI:10.1016/j.dyepig.2013.05.030 |
| [24] |
Janssens G, Touhari F, Gerritsen J W, Kempen H, Callant P, Deroover G, Vandenbroucke D. Chemical structure, aggregate structure and optical properties of adsorbed dye molecules investigated by scanning tunnelling microscopy[J]. Chemical Physics Letters, 2001, 344(1/2): 1-6. |
| [25] |
Hagfeldt A, Grätzel M. Light-induced redox reactions in nanocrystalline systems[J]. Chemical Reviews, 1995, 95(1): 49-68. DOI:10.1021/cr00033a003 |
| [26] |
Lenhard J R, Hein B R. Effects of J-aggregation on the redox levels of a cyanine dye[J]. Journal of Physical Chemistry, 1996, 100(43): 17287-17296. DOI:10.1021/jp961650l |
| [27] |
Xu W J, Zhang J B, Sun Y X, Ding B, Lin Y. Study on sulfuration of ZIF-67 thin film for counter electrode of squaraine-sensitized solar cell[J]. Imaging Science and Photochemistry, 2018, 36(5): 381-388. |
| [28] |
Bisquert J. Chemical capacitance of nanostructured semiconductors: its origin and significance for nanocomposite solar cells[J]. Physical Chemistry Chemical Physics, 2003, 5(24): 5360-5364. DOI:10.1039/b310907k |
| [29] |
Kabanakis A N, Bidikoudi M, Elsenety M M, Vougioukalakis G C, Falaras P. Synthesis of novel semi-squaraine derivatives and application in efficient dye-sensitized solar cells[J]. Dyes and Pigments, 2019, 165: 308-318. DOI:10.1016/j.dyepig.2019.02.028 |
| [30] |
Pei J, Feng K N, Wei Y N, Zhao X, Hao Y Z, Li Y P, Sun B, Chen S R, Lv H J. Influence of organic interface modification layer on the photoelectric properties of ZnO-based hybrid solar cells[J]. Journal of Photochemistry & Photobiology A: Chemistry, 2018, 364: 551-557. |
| [31] |
Rahman M U, Xie F Y, Li Y F, Wei M D. ZnO nano-sheets encapsulating graphene quantum dots with enhanced performance for dye-sensitized solar cell[J]. Journal of Electroanalytical Chemistry, 2019, 840: 160-164. DOI:10.1016/j.jelechem.2019.03.074 |
| [32] |
Liu Y M, Sun X H, Tai Q D, Hu H, Chen B L, Huang N, Sebo B, Zhao X Z. Influences on photovoltage performance by interfacial modification of FTO/mesoporous TiO2 using ZnO and TiO2 as the compact film[J]. Journal of Alloys and Compounds, 2011, 509(37): 9264-9270. DOI:10.1016/j.jallcom.2011.07.018 |
| [33] |
Cheng H, Wu Y G, Su J Y, Wang Z H, Ghimire R P, Liang M, Sun Z, Xue S. Organic dyes containing indolodithienopyrrole unit for dye-sensitized solar cells[J]. Dyes and Pigments, 2018, 149: 16-24. DOI:10.1016/j.dyepig.2017.09.053 |




