2. Hospital 904 of China Joint Logistic Support Force, Changzhou 213000, Jiangsu, P. R. China;
3. The First People's Hospital of Changzhou, Changzhou 213000, Jiangsu, P. R. China
2. 中国人民解放军联勤保障部队904医院, 江苏 常州 213000;
3. 常州第一人民医院, 江苏 常州 213000
Quantum dots (QDs), a type of semiconductor nanocrystals with the diameter of 1-100 nm, is getting more and more attention owing to the excellent optical properties[1, 2]. Up to now, it has extensively applied in various regions including fluorescence immunoassay[3], cell labeling and imaging[4] and in vivo imaging[5]. In recent decades, quantum dots, especially those with cadmium, have been widely studied. The traditional synthesis of QDs is carried out in organic phase or water phase, in which the potential toxicity of reagents and harsh preparation conditions limit their further application[6-8]. In the synthesis of QDs, it is necessary to add stabilizers to ensure their fluorescence efficiency and stability. The form of stabilizers directly affects the properties of QDs, even related to their toxicity, such as the release of heavy metals caused by the oxidation of mercapto stabilizers[9].
Biomimetic synthesis refers to a method which takes advantage of the self-assembling and molecular recognition ability of biomolecules or microorganisms to synthesize nanomaterials[10]. Compared with chemical synthesis, biomimetic technology has the advantages of environmental friendliness and good biocompatibility due to preparation in living cells. Since Sweeney firstly synthesized CdS quantum dots in Escherichia coli (E.coli)[11], scientists have gradually extended biomimetic synthesis to the synthesis of various quantum dots, most of which are synthesized by biotransformation of bacteria or fungi[12, 13]. The toxicity of cadmium containing quantum dots have been controversial for a long time. As a kind of nanomaterial with low toxicity, gold nanoparticles (Au NPs) have potential applications in medical diagnosis, treatment and compound detection. Many researchers use microorganisms to synthesize Au nanomaterials under relatively mild conditions[14, 15]. Their structures and optical properties are similar to those of chemical synthesis, but they have the advantages of lower toxicity.
As we all know, there are carbohydrates, lipids, proteins, nucleic acids and other bioactive substances in cells. Glutathione, a natural antioxidant in cells, is an important antidote for organisms. Various toxins, pollutants and carcinogens in human body can be discharged through the enzymatic channel of glutathione. In recent years, there have been many reports on the synthesis of nanoparticles due to the effect of glutathione in cell. Tan[16] synthesized near-infrared Ag2S quantum dots using glutathione as stabilizer in tumor cells. Anshup[17] synthesized Au nanoparticles in HEK-293 (human embryonic kidney), HeLa (human cervical cancer), SiHa (human cervical cancer), and SKNSH (human neuroblastoma) cells, in which they found that there were some differences, mainly in reducing capacity and cell metabolism between cancer cells and normal cells.
The surface properties of QDs are closely related to their optical properties. Changing the surface groups will lead to a sharp change in the fluorescence intensity of QDs. Results from the reduction properties of glutathione, glutathione-stabilized QDs can be used for detection of oxidative substances. In this work, driven by cell life metabolism, Au QDs was synthesized in living human breast cancer cell (MCF-7). The biosafety of the prepared Au QDs was compared with that of traditional chemical method. With the reductive properties of glutathione, the synthesis of Au QDs was used for the accurate detection of blood glucose concentration in diabetic rats, which provides reference for clinical application of glutathione stabilized QDs.
2 Materials and Methods 2.1 Chemical and MaterialsChloroauric acid, hydrogen peroxide and ethanol were all purchased from Sinopharm Chemical Reagent Co., Ltd. Cell Counting Kit-8 (CCK-8) purchased from Dojindo Molecular Technologies, Inc. RPMI-1640 cell culture medium, trypsin and RIPA cell lysate were purchased from Nanjing KeyGen Biotech Co., Ltd. All other reaction buffers and chemicals were purchased from Sinopharm Chemical Reagent Co., Ltd.
2.2 Cell CultureMCF-7 cells (human breast cancer cells) and HK-2 cells (human tubular epithelial cells, normal cells) were cultured in RPMI-1640 medium, HeLa (human cervical cancer cells) and CHL cells (lung cells, normal cells) were cultured in DMEM medium. Both two kinds of medium contained 10% fetal bovine serum, 100 U/mL penicillin and 100 g/mL streptomycin, cells cultured at regular cell culturing temperature of 37 ℃ and 5% CO2. Au QDs were synthesized in logarithmic growth cells with 80%~85% cell density.
2.3 Synthesis of Au QDs 2.3.1 Synthesis of Au QDs by Traditional Chemical Method2 mL 10 μmol/L glutathione aqueous solution and 2 mL 10 μmol/L HAuCl4 aqueous solution was added into a tin paper wrapped flask followed by diluting with 46 mL water to allow reacting for 2 h. Then, 2 mL 20 μmol/L NaBH4 solution was added to react for 3 h under rapidly stirring. At last, the reaction solution was purified with 2-fold volume of ethanol, then was centrifuged at 5000 rpm for 5 min. Au QDs solid powder was obtained by freeze-drying.
2.3.2 Synthesis of Au QDs in Living Tumor CellsChloroauric acid was dissolved in culture medium with a concentration of 1 mmol/L and filtered with 0.22 μm filter before use. MCF-7 cells were firstly washed twice by phosphate buffer solution (PBS) followed by respectively incubating with different concentration of chloroauric acid for 12, 24, 36, 48 and 60 h. The cells were washed twice with PBS and centrifuged for 5 min at 10000 rpm. Then 200 mL RIPA cell lysate was added. The cells were crushed by ultrasound and centrifuged for 5 min at 14000 rpm to obtain Au QDs solution. The purified Au QDs powder was prepared by precipitation and washing with isopropanol for three times.
2.4 Structure and Optical Characteristics of the Prepared Au QDsThe optical properties of Au QDs were characterized by fluorescence spectrophotometer. Transmission electron microscopy (TEM) and X-ray diffraction (XRD) were used to characterize its structure, particle size and dispersity. Au quantum dots were dispersed in water, PBS and cell culture media respectively. The stability of Au quantum dots was evaluated by measuring the fluorescence intensity at different time. The fluorescence efficiency was determined by comparative method[18].
2.5 Effects of Concentration and Incubation Time of Chloroauric AcidEffects of concentration and incubation time of chloroauric acid was evaluated by comparing the cell state and fluorescence intensity of Au QDs. Firstly, cells were seeded in a 96-well plate, followed by incubating cells in different concentrations of chloroauric acid for 24 h. The cells relative viabilities were evaluated by a cell titer 96 kit following vendor's protocols. After respectively incubating with 1 mmol/L chloroauric acid for 0, 12, 24, 36, 48 and 60 h, the cells state was observed and the optical properties of Au QDs were determined to evaluate the effect of incubation time.
2.6 Cytotoxicity Test of Au QDsCytotoxicity was tested by seeding cells in a 96-well plate, followed by Au QDs treatment. Incubating cells with different concentration of Au QDs including that prepared by traditional chemical method and biomimetic synthetic route. 24 h later, the cells relative viabilities were evaluated by a cell titer 96 kit following vendor's protocols.
2.7 Detection of Blood Glucose in Diabetic RatsDiabetic rats were operated under protocols approved by the Animal Ethics Committee of Xuzhou Medical University. All animal procedures were performed in accordance with the National Academy of Sciences Guide for the Care and Use of Laboratory Animals of USA[19]. Diabetic rats (n=6) were constructed by continuously giving high-fat and high-sugar diet for 4 weeks (containing 60% fat, 20% protein, 20% carbohydrate). Their blood glucose was measured before experiment, where a glucose level greater than 16.7 mmol/L was considered to be a successful model of rat diabetes[20]. The serum of diabetic rats was provided by the Pharmacology Laboratory of our university. 0.5 mmol/L Au QDs and 50 μg/mL glucose oxidase stock solution were prepared with PBS buffer respectively. A series of glucose standard solutions with different concentrations were diluted by PBS. In the determination process, 120 μL glucose oxidase and 20 μL Au QDs were added to 100 μL standard glucose solution (or rat serum sample) successively, and the volume was fixed to 100 mL with PBS. The fluorescence spectrum of the mixture was determined after incubation at 37℃ for 15 min.
3 Result and Discussion 3.1 Intracellular Synthesis and Characteristics of Au QDsA large amount of reducing substances such as glutathione and reducing sugar exist in cells, especially in cancer cells[21]. Herein, MCF-7 cancer cells were incubated with chloroauric acid, enabling gold ions to enter cells through ion channels on the cell membrane and react with the reductive substance in cells to form Au QDs (Figure 1a). After further extraction and purifi-cation, Au QDs powder was obtained.
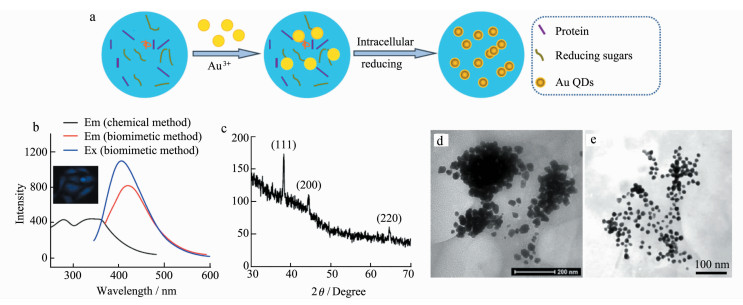
|
Figure 1 Biomimetic synthesis and optical and structural properties of Au QDs a. Schematic diagram of intracellular synthesis of Au QDs; b. Fluorescence spectra of Au QDs; c. XRD of Au QDs prepared by biomimetic method; d. TEM of Au QDs prepared by biomimetic method; e. TEM of Au QDs prepared by chemical method |
As shown in the up-left image in Figure 1b, MCF-7 cells emitted obvious blue fluorescence after incubation with chloroauric acid for 36 h. The extracted Au QDs was characterized by fluorescence spectra, X-ray diffraction (XRD) and transmission electron microscopy (TEM) measurement. With 350 nm as excitation wavelength, the Au QDs have an obvious emission peak at about 430 nm, and the emission peak is narrow and symmetrical, indicating that the size distribution of the prepared QDs is uniform. The excitation spectra of Au QDs show continuous absorption at 250-400 nm, which is consistent with the absorption characteristics of QDs. Compared with Rhodamine 6G (95%), the fluorescence efficiency of Au QDs respectively by chemical and intracellular method, was 8.4% and 6.1%. XRD results in Figure 1c showed that the synthesized QDs had three distinct diffraction peaks at 38.59, 46.37 and 64.65, which corresponded to the three crystal faces of (111), (200) and (220) in the standard card respectively. It was proved that the synthesized Au QDs had a cubic crystal structure. TEM images showed in Figure 1d indicated that the Au QDs synthesized by this method are approximately spherical, with a particle size of about 20 nm and good homogeneity, which was the same with that prepared by chemical method (in Figure 1e).
The fluorescence stability of Au QDs was also investigated. As shown in Figure 2, dispersed Au QDs in different medium including water, PBS and cell culture medium (RPMI-1640), their fluorescence intensity remained above 80.0% after two weeks, even above 69.5% after one month, which indicated that the synthesized Au QDs had good fluorescence stability and laid a foundation for later biological application.
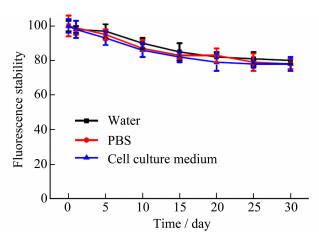
|
Figure 2 Fluorescence stability of Au QDs in different medium |
Chloroauric acid, as a chemical reagent, can affect the proliferation of cells, and then affect the intracellular synthesis of Au QDs. The effect of chloroauric acid concentration on cells viability and fluorescence (FL) intensity was studied in this work. As shown in Figure 3d, the cell viability decreased with the chloroauric acid concentration with a dose-dependent behavior. In order to study the influence of chloroauric acid concentration on the optical properties of quantum dots, chloroauric acid with five concentrations of 0.5 mmol/L, 1 mmol/L, 2 mmol/L, 4 mmol/L and 8 mmol/L were respectively investigated. Under the same conditions, the above chloroauric acid was respectively incubated with cells for 24 h, then Au QDs were extracted by cell lysis and their fluorescence properties were detected. As shown in Figure 3a and Figure 3b, the fluorescence intensity of Au QDs changes obviously with the increase of chloroauric acid concentration. When the concentration of chloroauric acid was in the range of 0-2 mmol/L, the fluorescence intensity gradually increased, while it decreased again with 4 mmol/L or high concentration. The cell morphology after incubation with chloroauric acid as shown in Figure 3c indicated that the long-term effect of foreign substances may reduce the normal metabolic activity of cells, thus hindering the effective synthesis of quantum dots in cells. Considering the morphology and fluorescence intensity of gold cells, the concentration of chloroauric acid was 2 mmol/L in the synthesis of Au QDs.

|
Figure 3 Effect of chloroauric acid concentration on fluorescence intensity (a and b) and cell viability (c and d) of Au QDs |
The incubation time is one of the key factors in the synthesis of Au QDs. The effect of incubation time of chloroauric acid and cells on the cell viability and fluorescence of gold quantum dots was studied in the range of 0-60 h with the chloroauric acid concentration of 2 mmol/L. As shown in Figure 4a and 4b, with the prolongation of co-incubation time between chloroauric acid and cells, the fluorescence intensity increased in the range of 0-36 h. The fluorescence intensity reached the maximum at 36 h. However, when incubation time continued to extend to 48 h, the fluorescence intensity decreased slightly. The reason for this result may be that the normal metabolic activity of cells was affected with the prolongation of incubation time after the gold ion enters the cells, resulting in cell damage and failure in synthesis. Figure 4c and 4d showed that with the prolongation of incubation time, the cell morphology gradually changes and cell viability decreased. When incubation time increased to 48 h, some cells became round and the intercellular space becomes larger, while the cell blurred and the boundary was not clear at 60 h. Considering the fluorescence intensity and cell state, the optimal incubation time was 36 h.
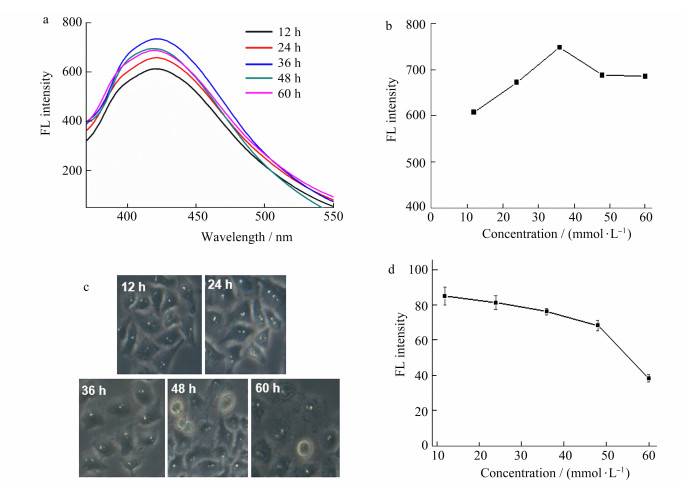
|
Figure 4 Effect of incubation time on fluorescence intensity (a and b) and cell viability (c and d) of Au QDs |
The cytotoxicity of Au QDs synthesized by chemical and biomimetic methods was investigated by comparing the cell survival rates of two kinds of cancer cells (HeLa cells and MCF-7 cells) and two kinds of normal cells (HK-2 cells and CHL cells). The concentration of QDs was set at low (20 μg/mL), medium (40 μg/mL) and high (60 μg/mL), and the incubation time with cells was 24 h. The toxicity of Au QDs to cells is time and dose dependent. The higher the dose, the higher the cell death rate. However, in biomimetic synthesis, the synthetic carrier is living cells and the reductive substance is endogenous substance. Therefore, Au QDs prepared by this method have better biocompatibility than that by traditional chemical methods. The survival rate of normal cells and tumor cells was still higher in 60 g/mL group after 24 h of exposure. As shown in Figure 5, after 24 h of incubation of Au QDs in high dose group with HeLa cells, the cell survival rate of chemical synthesis group was lower than 31.2%, while that of biomimetic group was 60.3%.
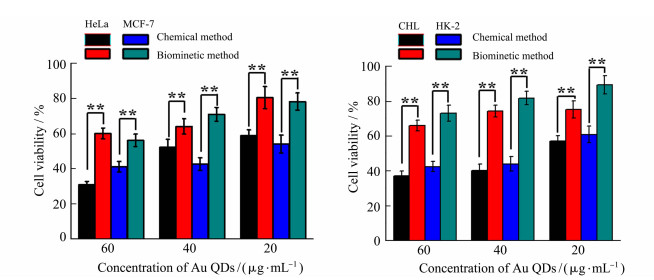
|
Figure 5 Cytotoxicity of Au QDs synthesized by different methods (**P < 0.01) |
The prepared Au QDs have good fluorescence properties. Because glucose can produce hydrogen peroxide under the catalysis of glucose oxidase, and hydrogen peroxide has strong oxidative properties, it can oxidize the surface of quantum dots to form new defects and finally lead to fluorescence quenching. Based on this, we can detect the concentration of glucose by monitoring the fluorescence intensity of Au QDs. The experimental results show that the fluorescence intensity of Au QDs decreases with the increase of glucose concentration (Figure 6). In the range of 7.85×10-6-2.5×10-4 mol/L, the fluorescence quenching efficiency of Au QDs (measured by(F0-F)/F0×100%) has a good linear relationship with the concentration of glucose with the detection limit of 3.1×10-8 mol/L. The recovery of this method is 90.0%-110.0%. Compared with traditional detection methods, this method has higher sensitivity and simpler and faster operation.
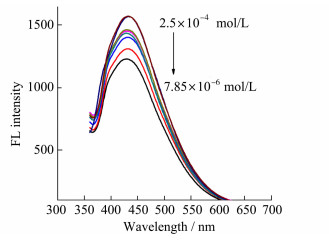
|
Figure 6 Effect of glucose on fluorescence properties of Au QDs |
The method was applied to the determination of serum glucose concentration which was compared with the commercial blood glucose monitor. The results show that the glucose concentration measured by this method is 23.97 mmol/L and the average concentration measured by the blood glucose monitor is 24.02 mmol/L. There is no obvious difference between the two results, which confirms the feasibility of this method.
There are two possible mechanisms for fluorescence quenching: one is that hydrogen peroxide produced during enzymatic reaction directly oxidizes the surface of Au QDs to form new defects which leads to fluorescence quenching[22], the other is that oxygen dioxide produced by the decomposition of hydrogen peroxide is a good electron acceptor, which is easy to form non-fluorescence anion on the surface of QDs[23]. Because glucose oxidase can only catalyze the oxidation of glucose and produce hydrogen peroxide with high specificity, the experimental results are not affected by other reducing sugars and have high selectivity.
4 ConclusionsIn this work, Au QDs were synthesized in MCF-7 cells. After cell lysis and extraction, the quantum dots were released from the cells. The Au QDs have good water solubility, good optical properties and strong stability. The cytotoxicity of Au QDs prepared by chemical and intracellular synthesis methods was compared. The results showed that there was a significant dose-dependent effect of Au QDs on the cytotoxicity of tumor cells and normal cells. The high dose group of Au QDs prepared by biomimetic method still maintained a high cell viability after incubation with cells for 24 h. The interaction between Au QDs and glucose oxidation was used to detect glucose. This method can be used to determine the content of blood glucose in diabetic rats without pretreatment. This method is simple and fast, and has some advantages in the field of medical application.
Acknowledgments We thank the Pharmacology Laboratory of our university for providing the serum of diabetic rats and helpful suggestions in experiments. This work is supported by Xuzhou Natural Science Foundation, China (KC18201, KC18108 and KC19066).| [1] |
Wagner A M, Knipe J M, Orive G, et al. Quantum dots in biomedical applications[J]. Acta Biomaterialia, 2019, 94: 44-63. DOI:10.1016/j.actbio.2019.05.022 |
| [2] |
Yao J, Li P, Li L, et al. Biochemistry and biomedicine of quantum dots:from biodetection to bioimaging, drug discovery, diagnostics, and therapy[J]. Acta Biomaterialia, 2018, 74: 36-55. DOI:10.1016/j.actbio.2018.05.004 |
| [3] |
Shah K G, Singh V, Kauffman P C, et al. Mobile phone ratiometric imaging enables highly sensitive fluorescence lateral flow immunoassays without external optical filters[J]. Analytical Chemistry, 2018, 90(11): 6967-6974. DOI:10.1021/acs.analchem.8b01241 |
| [4] |
Das P, Ganguly S, Maity P P, et al. Converting waste Allium sativum peel to nitrogen and sulphur co-doped photoluminescence carbon dots for solar conversion, cell labeling, and photobleaching diligences:a path from discarded waste to value-added products[J]. Journal of Photochemistry and Photo-biology B, 2019, 197: 111545. DOI:10.1016/j.jphotobiol.2019.111545 |
| [5] |
McHugh K J, Jing L, Behrens A M, et al. Biocompatible semiconductor quantum dots as cancer imaging agents[J]. Advanced Materials, 2018, 30(18): e1706356. DOI:10.1002/adma.201706356 |
| [6] |
Hossen S, Hossain M K, Basher M K, et al. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies:a review[J]. Journal of Advanced Research, 2018, 15: 1-18. |
| [7] |
Wang Y, Tang M. Review of in vitro toxicological research of quantum dot and potentially involved mechanisms[J]. Science of the Total Environment, 2018, 625: 940-962. DOI:10.1016/j.scitotenv.2017.12.334 |
| [8] |
Lu J, Tang M, Zhang T. Review of toxicological effect of quantum dots on the liver[J]. Journal of Applied Toxicology, 2019, 39(1): 72-86. DOI:10.1002/jat.3660 |
| [9] |
Sharma V K, McDonald T J, Sohn M, et al. Assessment of toxicity of selenium and cadmium selenium quantum dots:a review[J]. Chemosphere, 2017, 188: 403-413. DOI:10.1016/j.chemosphere.2017.08.130 |
| [10] |
Qi C, Musetti S, Fu L H, et al. Biomolecule-assisted green synthesis of nanostructured calcium phosphates and their biomedical applications[J]. Chemical Society Reviews, 2019, 48(10): 2698-2737. DOI:10.1039/C8CS00489G |
| [11] |
Sweeney R Y, Mao C, Gao X, et al. Bacterial biosynthesis of cadmium sulfide nanocrystals[J]. Chemistry & Biology, 2004, 11: 1553-1559. |
| [12] |
Ding Y, Bertram J R, Eckert C, et al. Nanorg microbial factories:light-driven renewable biochemical synthesis using quantum dot-bacteria nanobiohybrids[J]. Journal of the American Chemical Society, 2019, 141(26): 10272-10282. DOI:10.1021/jacs.9b02549 |
| [13] |
Qi S, Yang S, Chen J, et al. High-yield extracellular biosynthesis of ZnS quantum dots through a unique molecular mediation mechanism by the peculiar extracellular proteins secreted by a mixed sulfate reducing bacteria[J]. ACS Applied Materials & Interfaces, 2019, 11(11): 10442-10451. |
| [14] |
Xue J, Liu T, Liu Y, et al. Neuroprotective effect of biosynthesised gold nanoparticles synthesised from root extract of Paeonia moutan against Parkinson disease-In vitro & In vivo model[J]. Journal of Photochemistry and Photobiology. B, 2019, 200: 111635. DOI:10.1016/j.jphotobiol.2019.111635 |
| [15] |
Yan F, Liu L, Walsh T R, et al. Controlled synthesis of highly-branched plasmonic gold nanoparticles through peptoid engineering[J]. Nature Communications, 2018, 9(1): 2327. DOI:10.1038/s41467-018-04789-2 |
| [16] |
Tan L, Wan A, Li H. Synthesis of near-infrared quantum dots in cultured cancer cells[J]. ACS Applied Materials & Interfaces, 2014, 6(1): 18-23. |
| [17] |
Anshup A, Venkataraman J S, Subramaniam C, et al. Growth of gold nanoparticles in human cells[J]. Langmuir, 2005, 21(25): 11562-11567. DOI:10.1021/la0519249 |
| [18] |
Demas J N, Crosby G A. The Measurement of photoluminescence quantum yields[J]. Journal of Physical Chemistry, 1971, 75(8): 991-1024. DOI:10.1021/j100678a001 |
| [19] |
National Research Council. Guide for the Care and Use of Laboratory Animals[M]. Washington DC: National Academy Press, 2011: 3-4.
|
| [20] |
Srinivasan K, Ramarao P. Animal models in type 2 diabetes research:an overview[J]. Indian Journal of Medical Research, 2007, 125(3): 451-472. |
| [21] |
Ahamed M, Akhtar M J, Khan M A M, et al. Oxidative stress mediated cytotoxicity and apoptosis response of bismuth oxide (Bi2O3) nanoparticles in human breast cancer (MCF-7) cells[J]. Chemosphere, 2019, 216: 823-831. DOI:10.1016/j.chemosphere.2018.10.214 |
| [22] |
Shiang Y C, Huang C C, Chang H T. Gold nanodot-based luminescent sensor for the detection of hydrogen peroxide and glucose[J]. Chemical Communications, 2009, 23: 3437-3439. |
| [23] |
Cao L, Ye J, Tong L, et al. A new route to the considerable enhancement of glucose oxidase (GOx) activity:the simple assembly of a complex from CdTe quantum dots and GOx, and its glucose sensing[J]. Chemistry, 2008, 14(31): 9633-9640. DOI:10.1002/chem.200800681 |



