碳点,是一类尺寸在10 nm以下且形貌为类球形的具有特殊结构 (石墨烯、金刚石结构等) 的多色荧光新型碳纳米材料,也是继富勒烯、碳纳米管、石墨烯之后最受关注的新型碳纳米材料之一。它具有水溶性好、易表面修饰、生物毒性低、生物相容性好,以及发光性能稳定等一系列优点,在生物医学、环境能源和催化等领域有着重要的研究应用前景[1]。而且,碳点因其发光范围可调的突出优点使其在光电子器件、生物传感等方面具有潜在的应用价值。
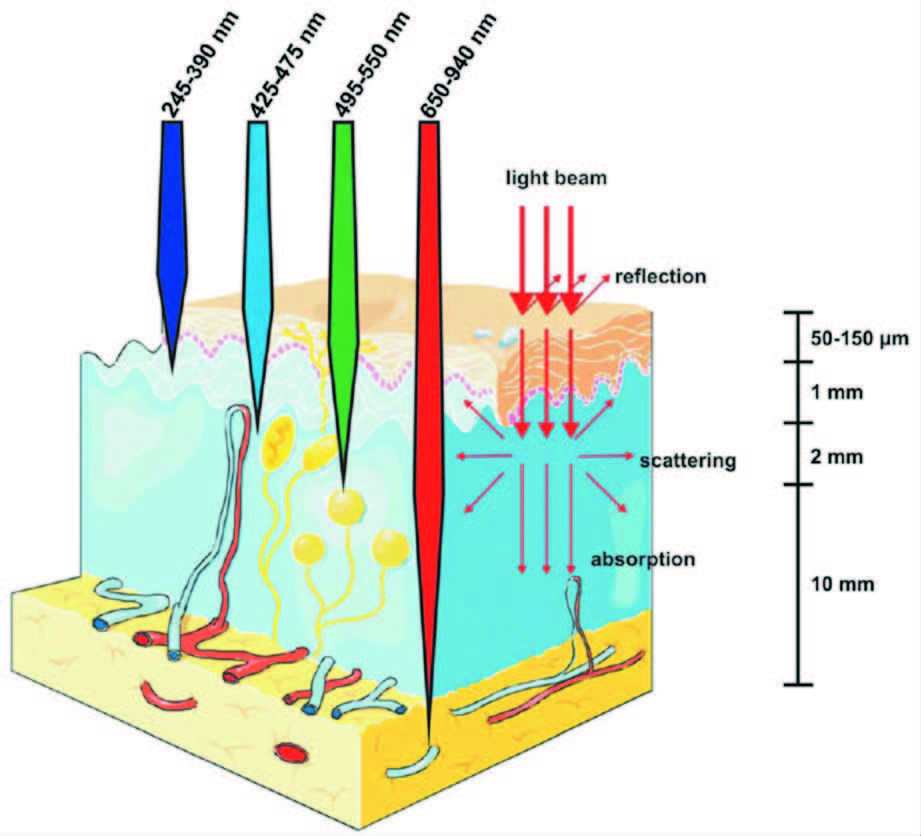
2004年,Scrivens研究组通过电弧放电的方法制备碳纳米管时第一次发现了荧光碎片。2006年,美国克莱蒙森大学孙亚平博士最先提出了碳点(碳量子点)的概念[2-5]。目前,碳点的制备方法主要分为自下而上法和自上而下法[6-8]。自下而上法是以有机分子或含有有机分子的生物质等为前驱物结合多种技术,如模板法[9, 10]、热解法[11-16]、微波法[17-20]、溶剂热法[21-26]、燃烧法[27-33]等将有机分子或含有有机分子的生物质碳化后,经过分离纯化得到发光碳点,这种方法产率高,但所得到的碳点发光大都在蓝绿光区域。自上而下法是把原来不发光的、大的块体碳材料,如石墨、碳纤维、碳纳米管、石墨烯等,利用多种技术如激光烧蚀[34-36]、电化学[37-42]、电弧放电[2]等制备尺寸更小的发光碳点,这类方法所得的碳点副产物多,产率较低,发光也大都在蓝绿光区域。但是,在实际应用尤其是生物医学应用研究中,蓝绿光因其穿透生物组织深度浅(1~2 mm)、本身对生物组织有伤害等不足,限制了此类碳点在生物医学中的应用研究。而红光或近红外发光碳点因其穿透生物组织能力强(10~30 mm)、本身对生物组织没有伤害等优点而备受研究者的青睐。随着纳米科学和技术的发展,碳点的制备方法也在不断改进,尤其是近两年,多种红光或近红外发光的碳点被制备出来。
本文简述了近3年来各类代表性的碳点的制备方法,并主要介绍了红光或近红外发光碳点的制备新进展。
1 自下而上法 1.1 模板法模板法即是利用模板来合成碳点,这种方法可以较好地对高温处理的碳点的形貌和粒径进行控制,得到的碳点均匀且不易团聚。但是由于碳点的生成是在模板上进行的,因而在去除模板时,碳点和模板的分离相对困难,而且在除去模板的同时会造成碳点的荧光以及形貌和粒径等性质发生变化,最终导致产率较低。
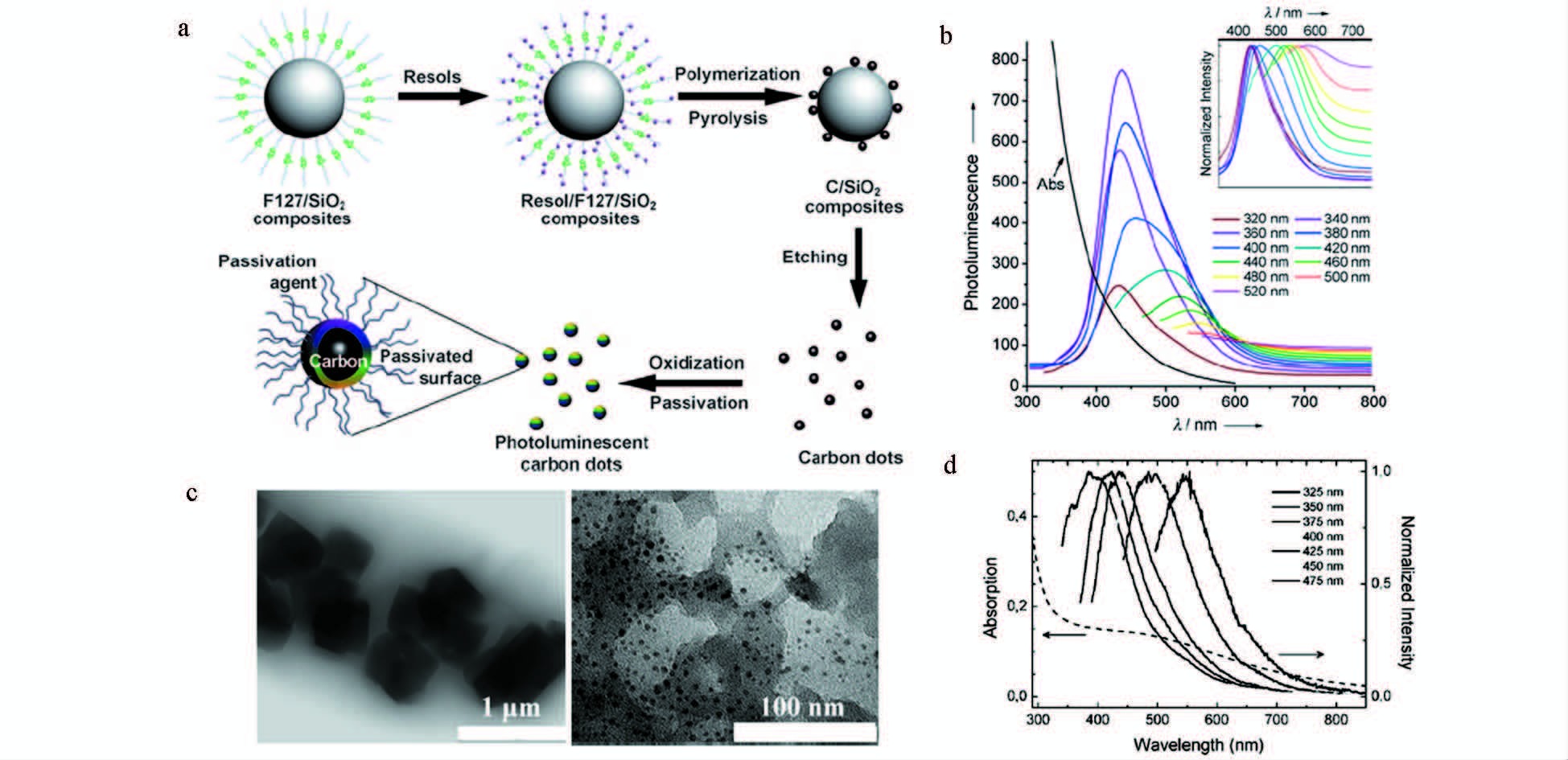
Liu研究组[9]通过将两性聚合物F127对二氧化硅纳米球进行功能化形成卫星状的聚合物F127/二氧化硅载体,然后将可溶性的酚醛树脂作为有机碳源,把聚合物F127/二氧化硅载体与碳源进行高温处理并移除二氧化硅模板,进一步酸化和表面钝化之后,最终形成的碳点粒径在1.5~2.5 nm,发光量子效率为14.7% (图 1a、1b)。Bourlinos等[10]利用被2,4-二氨基苯酚处理过的NaY沸石对其热氧化而后酸性刻蚀去除沸石模板制备出了粒径在4~6 nm的绿色荧光碳点 (图 1c、1d)。
|
图 1 模板法制备碳点 a.合成多色荧光碳点流程图[9];b.PEG1500N钝化后的吸收和荧光光谱图[9];c.刻蚀沸石之前 (左) 以及之后 (右) 的碳点的TEM示意图[10];d.吸收和荧光光谱图[10] Fig.1 Synthesize carbon dots by template method a.Processing diagram for the synthesis of multicolor photoluminescent carbon dots[9]; b.Optical spectra of absorption and photoluminescence after surface-passivated with PEG1500N[9]; c.TEM images before (left) and after (right) etching zeolite[10]; d.Optical spectra of absorption and photoluminescence[10] |
热解法是通过有机物碳源置于高温环境下将其碳化而制备碳点的一类方法。由于有机物碳源来源广泛,高温下的分解程度受到控制,因此这类方法具有操作方法简便、重复率高、可控性好的优点,并且制备出的碳点在可见光区具有宽的吸收谱图和高的荧光量子产率。
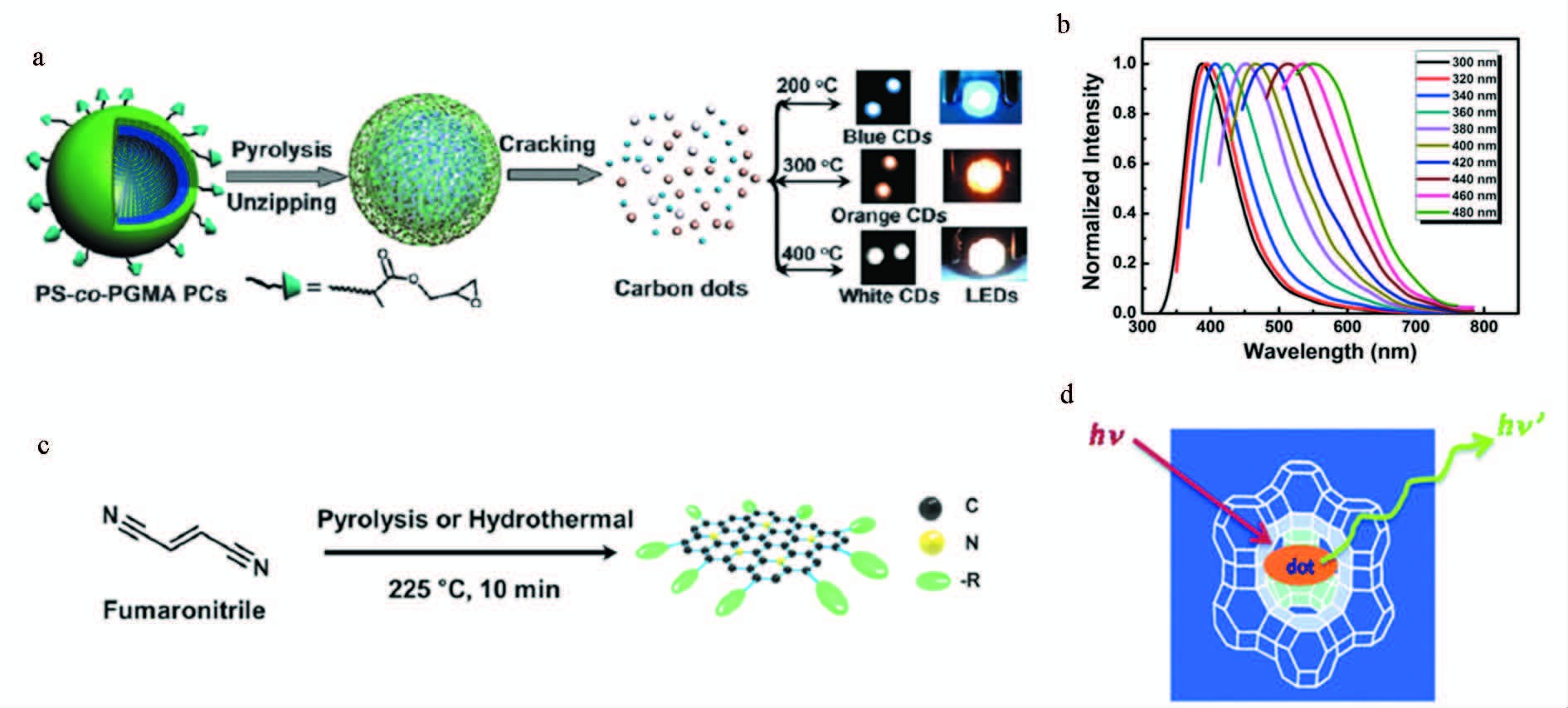
Guo等[11]通过控制热解温度分解由环氧聚苯乙烯与甲基丙烯酸缩水甘油酯交联制备的微球制备碳点,得到了多种荧光颜色的碳点,且荧光量子效率可达47%,此类高效发光碳点在光电子器件、传感、生物成像方面都具有十分重要的应用 (图 2a、2b)。Moon等[12]使用反丁烯二腈作为前驱体,高温热解一步生成粒径为3~6 nm,具有高效发光以及双亲特性的碳点,在光伏器件中可到达8.6%的光电转换效率 (图 2c)。Baldovi等[13]将有机物碳源的合成控制在具有纳米尺度孔洞的沸石中,进一步热解得到了尺寸可控的多色荧光碳点,并进一步将该类荧光碳点用到氧气传感器研究中(图 2d)。
|
图 2 热解法制备碳点 a.图示热解光学晶体形成碳点过程[11];b.碳点荧光光谱图[11];c.图示制备荧光碳点示例图[12];d.碳点合成原理图[13] Fig.2 Synthesize carbon dots by pyrolysis method a.Schematic representation of the formation of carbon dots from the pyrolysis of photonic crystals[11]; b.Photoluminescence spectra[11]; c.Schematic diagram for the preparation of photoluminescent carbon dots[12]; d.Schematic synthesis of carbon dots[13] |
微波法是利用微波加热将有机物碳化制备碳点的方法。微波法所需要的实验条件简便,且实验流程短节约时间,是潜在的适合工业化制备碳点的方法。其缺点在于不能有效控制碳点的生成,对其进一步表面功能化存在一定的难度。
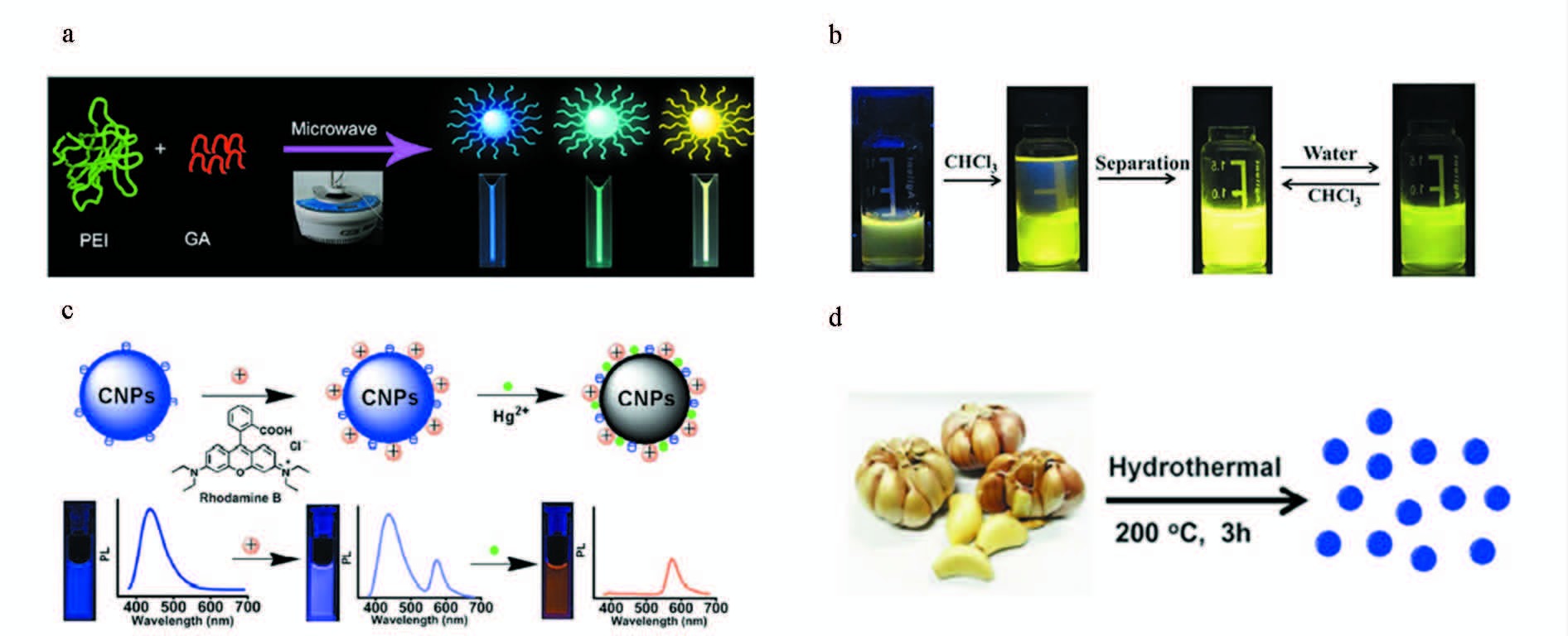
Liu等[17]利用聚乙烯亚胺微波(200 W,180 ℃)加热0.5 h制备出了蓝光碳点。然后,将聚乙烯亚胺和戊二醛混合,在相同条件下制备出了黄光碳点,该碳点在464~556 nm发射范围内,荧光可调,且具有制备方法简便、毒性低、稳定性好等优点,适于工业生产和细胞成像以及Co2+ 的检测(图 3a)。Xu等[18]利用葡萄糖作为前驱物在磷酸中快速微波热解形成具有双亲特性的黄光碳点,在三氯甲烷和水溶液中的荧光量子产率分别是11.5%和7.6%,可用于Cr离子的检测(图 3b)。Lan等[19]通过微波法将三聚氰胺和柠檬酸三钠碳化后制备出蓝光碳点,随后利用荧光变化机制设计了一种Hg2+离子纳米传感器(图 3c)。Zhao等[20]利用大蒜作为前驱体,通过体微波加热合成了荧光量子产率为17.5%的强蓝光碳点,该碳点既可用作荧光探针,又可用作自由基清除剂(图 3d)。
|
图 3 微波法制备碳点 a.微波热解形成多色碳点过程[17]; b.黄光碳点的纯化步骤[18]; c.CNPs-RhB纳米杂化系统的原理插图[19]; d.大蒜制备碳点的步骤[20] Fig.3 Synthesize carbon dots by microwave method a.Microwave pyrolysis approach to colorful carbon dots[17]; b.Purification procedure for the fluorescent Y-CDs[18];c.Schematic preparation of CNPs-RhB nanohybrid system[19]; d.Illustration of formation process of carbon dots from garlic[20] |
溶剂热法是将有机或无机碳源和水或有机溶剂混合后,转入高压反应釜中,利用加热过程中反应釜内产生的高温高压来制备碳点的一种方法。该方法好处在于一步即可将碳源碳化生成高荧光量子产率且能够在表面带有功能化基团的碳点,制备方法简便,适合于工业生产。
Yang等[21]报道了一种用水热法制备的N、S、Se元素掺杂荧光碳点,尺寸为1~6 nm左右,在蓝光-黄光范围内发光可调,可用作Cu2+和Hg2+离子的检测 (图 4a)。Li等[22]利用α-环糊精为碳源,在水热反应釜中制备了粒径为2 nm左右,荧光量子产率约为2.6%,且对血细胞有响应的蓝光碳点 (图 4b)。Jiang等[23]利用溶剂热法合成了粒径在10~18 nm,且在长波长荧光下具有高荧光量子产率(甲醇中:32.5%;去离子水中:10.8%)的黄光碳点,这类碳点通过结合Ag离子或者Cys可实现荧光的变化作为双功能传感器(图 4c)。Wang等[25]利用水热碳化技术将N-乙烯基咪唑制备出荧光量子产率为8%,且具有良好催化活性的蓝光碳点(图 4d)。

|
图 4 溶剂热法制备碳点 a.制备过程原理图[21];b.荧光碳点合成图[22];c.基于黄光碳点的双功能响应平台原理图[23]; d.水热合成碳点前后示意图及荧光光谱图[25] Fig.4 Synthesize carbon dots by solvothermal method a. Schematic diagram of the preparation process[21]; b. Synthesis of the fluorescent carbon dots[22]; c. Schematic illustration of the yellow-CDs-based bifunctional sensing platform[23]; d.Scheme for the photographs before and after the hydrothermal reaction and its photoluminescence spectra[25] |
燃烧法是一类较为简单的方法,利用蜡烛灰、石蜡灰、煤灰等即可获得碳点。该法所获得的碳点产量高,无需进一步钝化就可以发出荧光,但荧光量子产率较低。
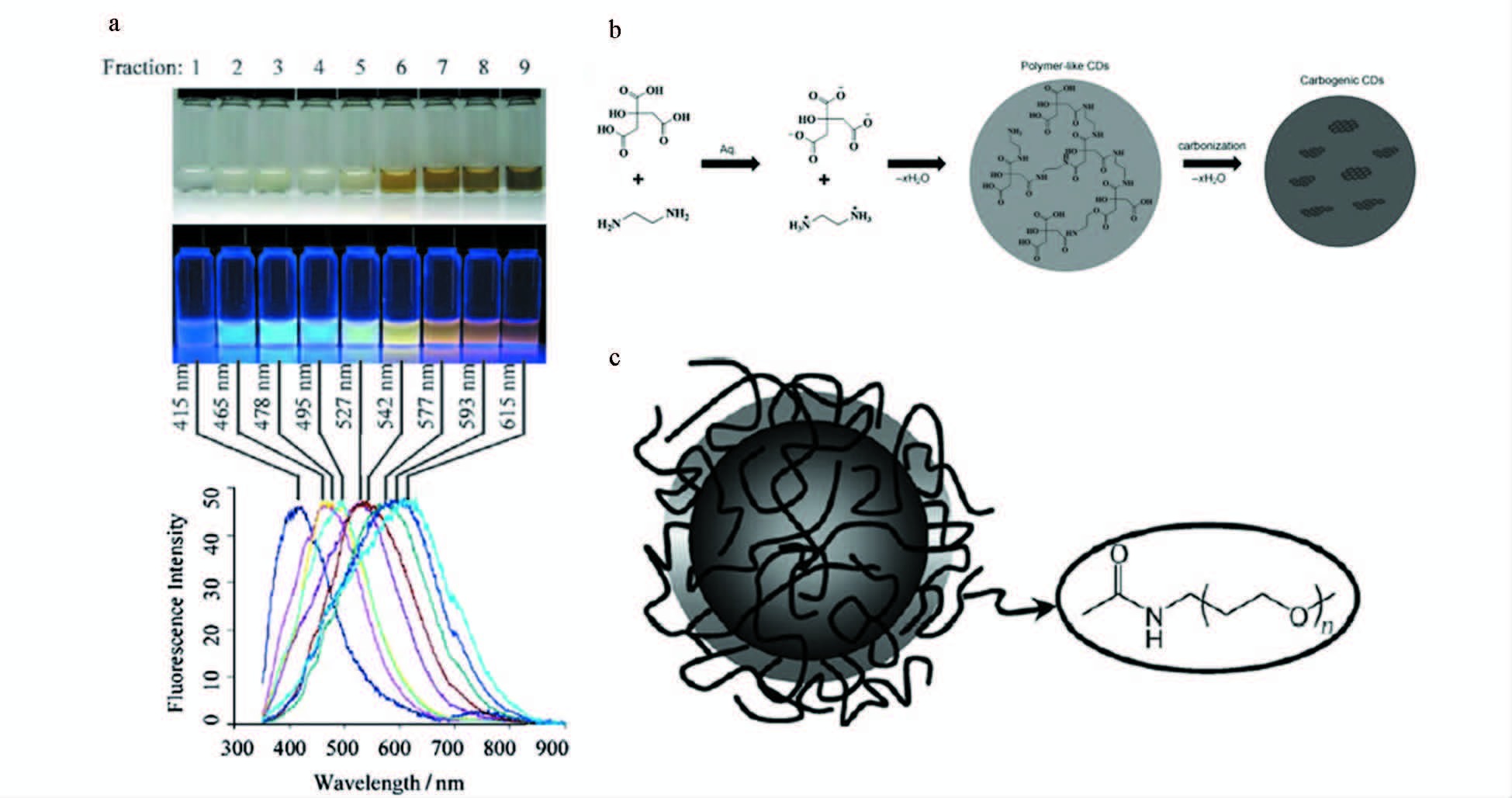
Liu等[31]第一次提出了用收集的蜡烛灰来制备碳点。他们把收集起来的炭黑用强氧化性酸硝酸进行氧化回流冷却,而后进行离心透析等手段最终得到在水中分散性较好的荧光碳点。这种方法结合电化学方法进行电泳分离可得到粒径约2 nm的多色发光碳点,其荧光量子产率仅为0.8%~1.8%(图 5a)。另外,石蜡油[32]以及秸秆[33]等燃烧形成的灰,经过强酸氧化也能形成多色高荧光量子产率的碳点 (图 5b、5c)。
|
图 5 燃烧法制备碳点 a.白光照射下的光学图像(上端),紫外光 (312 nm,中心) ,底端:碳纳米粒子溶液的荧光发射谱图 (315 nm激发)[31];b.石蜡油制备碳点机理图[32];c.秸秆制备碳点进一步钝化[33] Fig.5 Synthesize carbon dots by combustion method a.Optical images illuminated under white (top) and UV light (312 nm; center),bottom: fluorescence emission spectra (excitation at 315 nm) of the corresponding carbon nanoparticle solution[31]; b.Schematic preparation of carbon dots by paraffin[32]; c.Preparation of carbon dots for further passivating[33] |
激光烧蚀法是一类利用高能量的激光照射大的块体碳材料,将小的碳颗粒从碳材料上剥离出来形成碳点。这种方法是较早发现的一种方法,需要经过激光照射、氧化、钝化过程,操作繁琐,制备的碳点产率和纯度不高,粒径也不均匀。
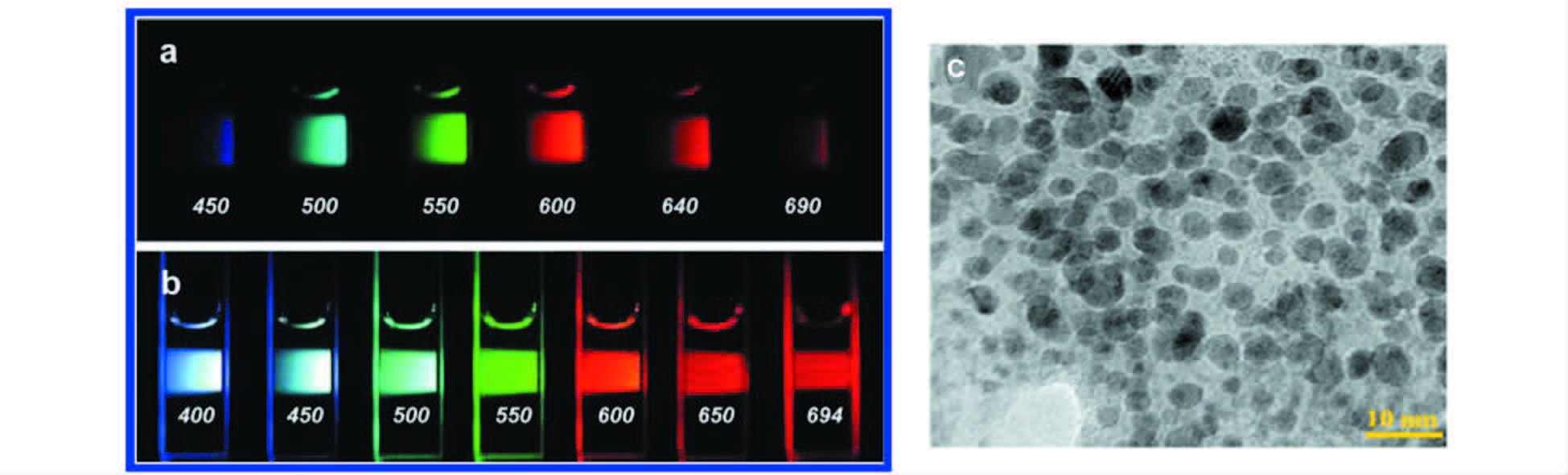
Sun等[5]将石墨粉作为碳源,氩气和水蒸气作为保护气,用Nd : YAG激光(1064 nm,10 Hz) 对其照射,用2.6 mol/L的硝酸溶液处理得到粒径3~12 nm的碳纳米颗粒,然后用PEG1500N或PPEI-EI对其钝化、透析后得到的多色发光碳点(图 6a、6b)。Russo等[34]利用飞秒激光器从块状石墨烯上剥离出尺寸在2~5 nm,荧光量子产率约为2%的石墨烯量子点(图 6c)。
|
图 6 激光烧蚀法制备碳点 PEG1500N连接的水相碳点溶液[5],a. 400 nm激发通过带通滤波器的不同波长的图;b. 按显示的波长激发并直接拍照;c.飞秒激光器制备的透射电镜图[34] Fig.6 Synthesize carbon dots by laser ablation method Aqueous solution of the PEG1500N-attached carbon dots[5],a. excited at 400 nm and photographed through band-pass filters of different wavelengths as indicated; b. excited at the indicated wavelengths and photographed directly; c. TEM image of GQDs prepared by femtosecond laser[34] |
电化学法是通过电解石墨、多壁碳纳米管等块体材料来制备碳点的方法。电化学法制备碳点可以通过准确控制电极两端的电压和电流强度,进而控制碳点的制备,而且该法的容易操作、制备成本廉价、产量高。但也同样存在着碳点产物荧光量子产率较低、不易进一步功能化等缺点。
Niu等[37]利用乙腈、离子液体等在不同比例下通过电极作用制备出了粒径约3 nm,不同荧光量子产率的蓝光碳点 (图 7a)。Shih等[38]通过控制温度和时间制备出的多颜色的SiC-dots (图 7b),这类碳点具有很强的电催化活性。Zheng等[41, 42]利用石墨和Pt作为正反电极,Ag/AgCl作为参比电极,中性磷酸盐溶液 (PBS) 作为电解质,通过控制电压在-3~3 V之间变化,制备出了尺寸在2~20 nm之间的多色发光碳点 (图 7c)。

|
图 7 电化学法制备碳点 a. 电化学法自上而下制备碳点的机制[37]; b. 多色纳米复合物粉末及其不同激发下的荧光[38]; c.石墨棒制备产生的电化学荧光[41, 42] Fig.7 Synthesize carbon dots by electrochemical method a.Mechanism of top-down EC strategy for carbon dots generation[37]; b.Photographs of multi-color nanocomposite powders and fluorescence under various excitation[38]; c.Synthesize from a graphite rod which are capable of electrochemiluminescence (ECL)[41, 42] |
电弧放电法是利用高压电弧放电剥离出碳点的一类方法。由于碳点来源于电弧放电所产生的成分复杂的碳灰,存在提纯困难和产率低的缺点,但经过强酸强碱处理后即可发出荧光。
Su等[43]将电弧放电制备方法与离心分离和化学氧化方法结合起来,制备出了粒径分布较窄(3.2~8 nm)、荧光量子产率为3.31%,并且具有上转换荧光的碳点,这类碳点也表现出了优异的光电特性(图 8a)。Xu等[44]利用电弧放电合成方法制备出了碳点,并首次利用碳点作为发色团捕获可见光光子,实现能量转换(图 8b)。

|
图 8 电弧放电法制备碳点 a. 电弧放电合成荧光碳点的步骤[43];b. 碳点的透射电镜和扫描电镜图[44] Fig.8 Synthesize carbon dots by arc discharge method a. Synthetic procedure of fluorescent CDs using arc-synthesized method[43]; b. Photographs of carbon dots by TEM and SEM[44] |
以上介绍了两类主要的自下而上和自上而下的制备碳点的方法,但是无论用哪类方法,得到的大都是蓝绿光碳点。在2014年以前,红光或近红外发光的碳点的制备很少有报道。但在实际应用尤其是生物医学应用研究中,蓝绿光碳点因其穿透生物组织浅(1~2 mm)、本身对生物组织有伤害等不足,限制了此类碳点在生物医学中的应用研究。而红光或近红外光发光的碳点,因其穿透生物组织能力强(10~30 mm)、本身对生物组织无伤害等优点而备受研究者的青睐(图 9)[45]。
近年来,多个课题组改进了以前用单个有机分子为碳源的制备方法,采用多种有机分子混合或含杂原子的聚合物为前驱物,结合高温碳化技术,制备了多种红光或近红外荧光的碳点(图 10)。如:Zheng等[46]用花菁染料和聚乙二醇800为前驱物,用水热碳化技术制备了发光在600~900 nm,最高发射峰为800 nm左右的近红外发光碳点,实现了细胞水平和活体水平的荧光成像。Fan等[47]用石墨为碳源,以过二硫酸钾为氧化剂,采用电化学刻蚀的方法,通过改变电化学刻蚀时间,调控碳点的发光,制备出了最高发射峰为630 nm的红光碳点,用于细胞成像。Hu等[48]利用聚乙二醇、乙二胺-聚乙烯亚胺的混合物为碳源,和硼氢化钠或磷酸混合,然后利用高温碳化技术,在180 ℃的高温下制备了发光范围在530~710 nm之间的红光碳点,并研究了碳点的发光机理。Jiang等[49]直接用对苯二胺为碳源,利用溶剂热反应是为碳化对苯二胺,制备出了最大发射峰为620 nm的红光碳点,用于细胞成像。Jiang等[50]用磷酸和硫酸的混合物为强氧化剂,100 ℃反应5 h,制备了最高发射峰为620 nm的红光碳点,用于氨气的检测。

|
图 10 红光或近红外发光碳点的制备 a. 红光碳点的制备流程图[46];b. 360 nm激发下的不同时间下的氧化石墨烯量子点的荧光光谱[47];e.由甘蔗渣合成碳点的原理示意图[50] Fig.10 Synthesis of red-emissive or near-infrared emissive carbon dots a. Illustration of preparation of red fluorescent carbon dots[46]; b. Normalized photoluminescence spectra of progressively oxidized graphene quantum dots at different times under 360 nm laser excitation[47]; c.Synthesis of carbon dots with different emission colors[48]; d. Preparation of the RGB photoluminescence carbon dots from three isomers(i.e. oPD,mPD,pPD) and fluorescence photographs under 365 nm irradiation[49]; e. Schematic illustration of the synthetic procedure for obtaining carbon dots from bagasse[50] |
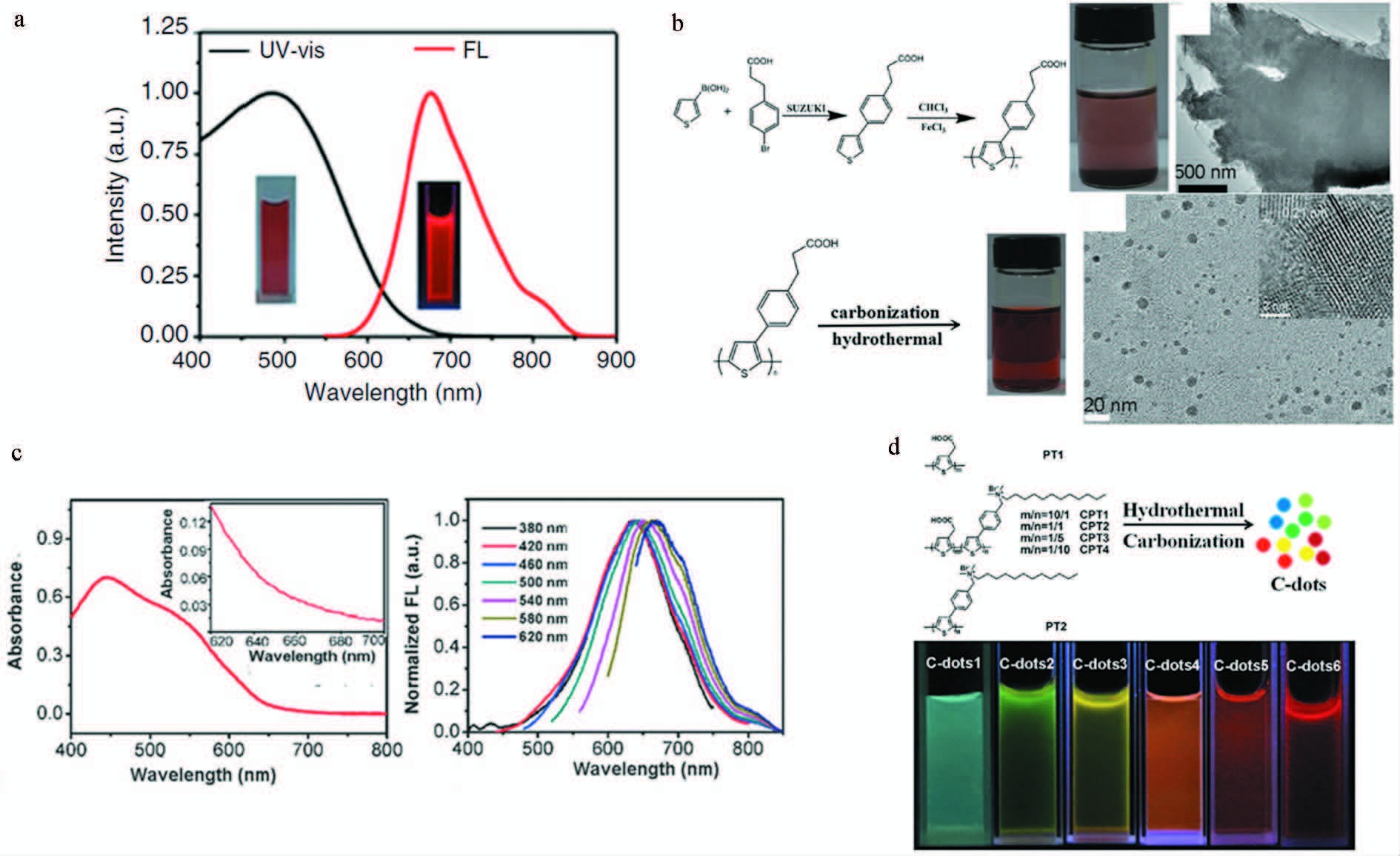
最近,我们课题组发展了一种制备红光或近红外发光碳点的新方法(图 11)。我们首先以单个含有杂原子的聚合物聚噻吩季铵盐[51]、聚噻吩苯丙酸[52]和聚噻吩苯甲酸[53]等含S或同时含有S和N的聚噻吩衍生物为碳源,结合水热碳化技术,制备出了3种红光或近红外发光碳点,用作荧光成像介导的肿瘤光动力或光热治疗。随后,进一步调控聚噻吩季铵盐和聚噻吩乙酸的比例,生成多种共聚物,以这些共聚物为碳源,结合水热碳化技术,制备出了从绿光到近红外发光的一系列碳点,用于细胞的多色成像[54]。
|
图 11 聚噻吩衍生物为前驱物制备红光碳点 a. 归一化紫外-可见吸收和500 nm激发光谱以及365 nm激发下的荧光图片[51];b. 合成碳点的路径和透射电镜图片[52];c. 紫外-可见吸收及其多波长激发下的荧光[53];d.碳点的合成路径及其紫外光激发下的不同颜色[54] Fig.11 Polythiophene derivatives as precursors to synthesize red-emissive carbon dots a. Normalized UV-Vis absorption and emission spectra (λ=500 nm) of the graphene quantum dots and photographs of fluorescence under 365 nm excitation[51]; b. Synthetic route of carbon dots and its TEM images[52]; c. UV-Vis absorption and fluorescence under multi-excitation[53]; d.Synthetic routes of carbon dots and its various colors under UV light[54] |
传统的量子点尤其是Ⅱ-Ⅳ族含镉量子点,尽管发光可调,荧光量子产率高,被广泛用作纳米荧光探针以及生物体内的示踪、传感和荧光标记,但是重金属的潜在毒性已经引起了量子点研究者的高度重视。最有潜力代替这些传统量子点的是硅量子点和碳点(碳量子点),但是硅量子点不稳定,在空气中很容易氧化成二氧化硅失去荧光。而碳点除了具有硅量子点的优越特性以外,它在空气中不容易被氧化,光稳定性比较好。因此,荧光碳点尤其是红光和近红外发光的碳点成为了纳米生物荧光成像研究领域的重点研究对象。由于红光或近红外发光的碳点制备方法还处于摸索阶段,且目前发现的红光或近红外发光碳点的荧光量子产率很低(一般低于10%),和红光硅量子点的荧光量子产率比还有差距。因此,还需继续改进前躯体分子的设计,并不断探索碳点的表面钝化机制,以期制备出高荧光量子产率的红光或近红外发光碳点。另外,由于红光或近红外发光的碳点可吸收可见光甚至更长波长的光,在纳米光诊疗生物医学领域、水处理环境保护领域、量子点敏化太阳能电池、光催化合成化学品等众多研究领域具有重要的应用前景。因此,需要不断地探索新的碳源分子来制备红光和近红外发光碳点,实现其以上诸多领域的广泛应用。
| [1] | Zhao H X, Liu L Q, Liu Z D, Wang Y, Zhao X J, Huang C Z. Highly selective detection of phosphate in very complicated matrixes with an off-on fluorescent probe of europium-adjusted carbon dots[J]. Chemical Communications, 2011, 47 (9) :2604–2606. DOI:10.1039/c0cc04399k |
| [2] | Xu X Y, Ray R, Gu Y L, Ploehn H J, Gearheart L, Raker K, Scrivens W A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments[J]. Journal of the American Chemical Society, 2004, 126 (40) :12736–12737. DOI:10.1021/ja040082h |
| [3] |
王林鹏, 马玉洁, 周学华, 刘云, 武端东. 碳点的制备与应用研究进展[J]. 材料工程, 2015, 43 (5) :101–112.
Wang L P, Ma Y J, Zhou X H, L Y, Wu D D. Progress in research on preparation and application of carbon dots[J]. Journal of Materials Engineering, 2015, 43 (5) :101–112. |
| [4] |
王胜达, 袁楠, 朱志峰, 蒋阳. 荧光碳点的合成?性质和应用[J]. 影像科学与光化学, 2016, 34 (3) :203–218.
Wang S D, Yuan N, Zhu Z F, Jiang Y. Fluorescent carbon dots:synthesis, characterization and application[J]. Imaging Science and Photochemistry, 2016, 34 (3) :203–218. |
| [5] | Sun Y P, Zhou B, Lin Y, Wang W, Fernando K A, Pankaj P, Mohammed J M, Harruff B A, Wang X, Wang H F. Quantum-sized carbon dots for bright and colorful photoluminescence[J]. Journal of the American Chemical Society, 2006, 128 (24) :7756–7757. DOI:10.1021/ja062677d |
| [6] | Baker S N, Baker G A. Luminescent carbon nanodots:emergent nanolights[J]. Angewandte Chemie International Edition, 2010, 49 (38) :6726–6744. DOI:10.1002/anie.200906623 |
| [7] |
颜范勇, 邹宇, 王猛, 代林枫, 周旭光, 陈莉. 荧光碳点的制备及应用[J]. 化学进展, 2014, 26 (1) :61–74.
Yan F Y, Zhou Y, Wang M, Dai L F, Zhou X G, Chen L. Synthesis and application of the fluorescent carbon dots[J]. Progress in Chemistry, 2014, 26 (1) :61–74. |
| [8] | Xin Y, Xiao C, Liang S L. Synthesis of large, stable colloidal graphene quantum dots with tunable size[J]. Journal of the American Chemical Society, 2010, 132 :5944. DOI:10.1021/ja1009376 |
| [9] | Liu R L, Wu D Q, Liu S H, Koynov K, Knoll W, Li Q. An aqueous route to multicolor photoluminescent carbon dots using silica spheres as carriers[J]. Angewandte Chemie International Edition, 2009, 48 :4598–4601. DOI:10.1002/anie.v48:25 |
| [10] | Bourlinos A B, Stassinopoilos A, Anglos D, Zboril R, Georgakilas V, Giannelis E P. Photoluminescent carbogenic dots[J]. Chemistry of Materials, 2008, 20 (14) :4539–4541. DOI:10.1021/cm800506r |
| [11] | Guo X, Wang C F, Yu Z Y, Chen L, Chen S. Facile access to versatile fluorescent carbon dots toward light-emitting diodes[J]. Chemical Communications, 2012, 48 (21) :2692–2694. DOI:10.1039/c2cc17769b |
| [12] | Moon B J, Oh Y, Shin D H, Kim S J, Lee S H, Kim T, Park M, Bae S. Facile and purification-free synthesis of nitrogenated amphiphilic graphitic carbon dots[J]. Chemical Materials, 2016, 28 :1481–1488. DOI:10.1021/acs.chemmater.5b04915 |
| [13] | Baldovi H G, Valencia S, Alvaro M, Asiri A M, Garcia H. Highly fluorescent C-dots obtained by pyrolysis of quaternary ammonium ions trapped in all-silica ITQ-29 zeolite[J]. Nanoscale, 2015, 7 :1744–1752. DOI:10.1039/C4NR05295A |
| [14] | Stan C S, Albu C, Coroaba A, Popa M, Sutiman D. One step synthesis of fluorescent carbon dots through pyrolysis of N-hydroxysuccinimide[J]. Journal of Materials Chemistry C, 2015, 3 :789–795. |
| [15] | Mao L H, Tang W Q, Deng Z Y, Liu S S, Wang C F, Chen S. Facile access to white fluorescent carbon dots toward light-emitting devices[J]. Industrial & Engineering Chemistry Research, 2014, 53 (15) :6417–6425. |
| [16] | Xu H, Yang X P, Li G, Zhao C, Liao X J. Green synthesis of fluorescent carbon dots for selective detection of tartrazine in food samples[J]. Journal of Agricultural and Food Chemistry, 2015, 63 (30) :6707–6714. DOI:10.1021/acs.jafc.5b02319 |
| [17] | Liu H, He Z, Jiang L P, Zhu J J. Microwave assisted synthesis of wavelength tunable photoluminescent carbon nanodots and their potential application[J]. ACS Applied Material & Interface, 2015, 7 :4913–4920. |
| [18] | Xu Z Z, Wang C X, Jiang K L, Lin H H, Huang Y J, Zhang C. Microwave-assisted rapid synthesis of amphibious yellow fluorescent carbon dots as a colorimetric nanosensor for Cr(Ⅵ)[J]. Particle & Particle Systems Characterization, 2015, 32 :1058–1062. |
| [19] | Lan M H, Zhang J F, Chui Y S, Wang P F, Chen X F, Lee C S, Kwong H L, Zhang W J. Carbon nanoparticle-based ratiometric fluorescent sensor for detecting mercury ions in aqueous media and living cells[J]. ACS Applied Material & Interface, 2014, 6 (23) :21270–21278. |
| [20] | Zhao S J, Lan M H, Zhu X Y, Xue H T, Ng T W, Meng X M, Lee C S, Wang P F, Zhang W J. Green synthesis of bifunctional fluorescent carbon dots from garlic for cellular imaging and free radical scavenging[J]. ACS Applied Material & Interface, 2015, 7 (31) :17054–17060. |
| [21] | Yang S W, Sun J, Li X B, Zhou W, Wang Z Y, He P, Ding G Q, Xie X M, Kang Z H, Jiang M H. Large-scale fabrication of heavy doped carbon quantum dots with tunable-photoluminescence and sensitive fluorescence detection[J]. Journal of Materials Chemistry A, 2014, 2 :8660–8867. DOI:10.1039/c4ta00860j |
| [22] | Li S, Guo Z, Zhang Y, Xue W, Liu Z H. Blood compatibility evaluations of fluorescent carbon dots[J]. ACS Applied Material & Interface, 2015, 7 :19153–19162. |
| [23] | Jiang K, Sun S, Zhang L, Wang Y H, Cai C Z, Lin H W. Bright-yellow-emissive N-doped carbon dots:preparation, cellular imaging, and bifunctional sensing[J]. ACS Applied Material & Interface, 2015, 7 :23231–23238. |
| [24] | Zhu X H, Xiao X, Zuo X X, Liang Y, Nan J M. Hydrothermal Preparation of Photoluminescence graphene quantum dots characterized excitation-independent emission and its application as a bioimaging reagent[J]. Particle & Particle Systems Characterization, 2014, 31 (7) :801–809. |
| [25] | Wang B, Liu H J, Chen Y. A biocompatible poly (N-vinylimidazole)-dot with both strong luminescence and good catalytic activity[J]. RSC Advances, 2016, 6 :2141–2148. DOI:10.1039/C5RA20640E |
| [26] | Kasibabu B S B, D'souza S L, Jha S, Sinqhal R K, Basu H, Kailasa S K. One-step synthesis of fluorescent carbong dots for imaging bacterial and fungal cells[J]. Analytical Methods, 2015, 7 :2373–2378. DOI:10.1039/C4AY02737J |
| [27] | Ray S C, Saha A, Jana N R, Sarkar R. Fluorescent carbon nanoparticles:synthesis, characterization, and bioimaging application[J]. The Journal of Physical Chemistry C, 2009, 113 :18546–18551. DOI:10.1021/jp905912n |
| [28] | Vinci J C, Colon L A. Fractionation of carbon-based nanomaterials by anion exchange HPLC[J]. Analytical Chemi-stry, 2011, 84 (2) :1178–1183. |
| [29] | Tian L, Ghosh D, Chen W, Pradhan S, Chang X J, Chen S W. Nanosized carbon particles from natural gas soot[J]. Chemistry of Materials, 2009, 21 (13) :2803–2809. DOI:10.1021/cm900709w |
| [30] | Tang L B, Ji R B, Cao X K, Lin J Y, Jiang H X, Li X M, Teng K S, Luk C M, Zeng S J, Hao J H, Lau S P. Deep ultraviolet photoluminescence of water-soluble self-passivated graphene quantum dots[J]. ACS Nano, 2012, 6 (6) :5102–5110. DOI:10.1021/nn300760g |
| [31] | Liu H P, Ye T, Mao C D. Fluorescent carbon nanoparticles derived from candle soot[J]. Angewandte Chemie International Edition, 2007, 46 (34) :6473–6475. DOI:10.1002/(ISSN)1521-3773 |
| [32] | Zhu S J, Meng Q N, Wang L, Zhang J H, Song Y B, Jin H, Zhang K, Sun H C, Wang H Y, Yang B. Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging[J]. Angewandte Chemie International Edition, 2013, 125 (14) :4045–4049. |
| [33] | Wang X, Cao L, Yang S T, Lu F S, Meziani M J, Tian L L, Sun K W, Bloodgood M A, Sun Y P. Bandgap-Like Strong Fluorescence in Funcationalized Carbon Nanoparticles[J]. Angewandte Chemie International Edition, 2010, 49 (31) :5310–5314. DOI:10.1002/anie.v49:31 |
| [34] | Russo P, Liang R, Jabari E, Marzbanrad E, Toyserkani E, Zhou Y N. Single-step synthesis of graphene quantum dots by femtosecond laser ablation of graphene oxide dispersions[J]. Nanoscale, 2016, 8 :8863–8877. DOI:10.1039/C6NR01148A |
| [35] | Yang S T, Wang X, Wang H F, Lu F S, Luo P G, Cao L, Meziani M J, Liu J H, Liu Y F, Chen M, Huang Y P, Sun Y P. Carbon dots as nontoxic and high-performance fluorescence imaging agents[J]. The Journal of Physical Chemistry C, 2009, 113 (42) :18110–18114. DOI:10.1021/jp9085969 |
| [36] | Russo P, Hu A, Compaqnini G, Duley W W, Zhou N Y. Femtosecond laser ablation of highly oriented pyrolytic graphite:a green route for large-scale production of porous graphene and graphene quantum dots[J]. Nanoscale, 2014, 6 :2381–2389. DOI:10.1039/c3nr05572h |
| [37] | Niu F S, Xu Y H, Liu M L, Sun J, Guo P R, Liu J Q. Bottom-up electrochemical preparation of solid-state carbon nanodots directly from nitriles/ionic liquids using carbon-free electrodes and the applications in specific ferric ion detection and cell imaging[J]. Nanoscale, 2016, 8 :5470–5477. DOI:10.1039/C6NR00023A |
| [38] | Shih C C, Chen P C, Lin G L, Wang C W, Chang H T. Optical and electrochemical applications of silicon-carbon dots/silicon dioxide nanocomposites[J]. ACS Nano, 2015, 9 :312–319. DOI:10.1021/nn504787y |
| [39] | Li H T, He X D, Kang Z H, Huang H, Liu Y, Liu J L, Lian S Y, Tsang C H A, Yang X B, Lee S T. Water-soluble fluorescent carbon quantum dots and photocatalyst design[J]. Angewandte Chemie International Edition, 2010, 49 (26) :4430–4434. DOI:10.1002/anie.200906154 |
| [40] | Ming H, Ma Z, Liu Y, Pan K, Yu H, Wang F, Kang Z H. Large scale electrochemical synthesis of high quality carbon nanodots and their photocatalytic property[J]. Dalton Transactions, 2012, 41 (31) :9526–9531. DOI:10.1039/c2dt30985h |
| [41] | Zheng L Y, Chi Y W, Dong Y Q, Lin J P, Wang B B. Electrochemiluminescence of water-soluble carbon nanocrystals released electrochemically from graphite[J]. Journal of the American Chemical Society, 2009, 131 :4564–4565. DOI:10.1021/ja809073f |
| [42] |
闵云浩.荧光碳点的合成制备及其性能研究[D]. 广东:华南理工大学,2014.
Min Y H. Synthesis and performance research of Carbon dots[D]. Guangdong:South China University of Technology, 2014. |
| [43] | Su Y J, Xie M M, Lu X N, Wei H, Geng H J, Yang Z, Zhang Y F. Facile synthesis and photoelectric properties of carbon dots with upconversion fluorescence using arc-synthesized carbon by-products[J]. RSC Advances, 2014, 4 :4839–4842. DOI:10.1039/c3ra45453c |
| [44] | Xu J, Sahu S, Cao L, Anilkumar P, Tacket K N, Qian H J, Bunker C E, Guliants E A, Parenzan A, Sun Y P. Carbon nanoparticles as chromophores for photon harvesting and photoconversion[J]. ChemPhysChem, 2011, 12 (18) :3604–3608. DOI:10.1002/cphc.v12.18 |
| [45] | Zhou Z J, Song J B, Nie L M, Chen X Y. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy[J]. Chemical Society Reviews, 2016 . DOI:10.1039/C6CS00271D |
| [46] | Zheng M, Li Y, Liu S, Wang W Q, Xie Z G, Jing X B. One-Pot To Synthesize Multifunctional Carbon Dots for Near Infrared Fluorescence Imaging and Photothermal Cancer Therapy[J]. ACS Applied Material & Interface, 2016, 8 :23533–23541. |
| [47] | Tan X Y, Li Y C, Li X H, Zhou S X, Fan L Z, Yang S H. Electrochemical synthesis of small-sized red fluorescent graphene quantum dots as a bioimaging platform[J]. Chemical Communications, 2015, 51 :2544–2546. DOI:10.1039/C4CC09332A |
| [48] | Hu S L, Trinchi A, Atkinl P, Cole I. Tunable photoluminescence across the entire visible spectrum from carbon dots excited by white light[J]. Angewandte Chemie International Edition, 2015, 54 :2970–2974. DOI:10.1002/anie.201411004 |
| [49] | Jiang K, Sun S, Zhang L, Lu Y, Wu A, Cai C Z, Lin H W. Red, green, and blue luminescence by carbon dots:full-color emission tuning and multicolor cellular imaging[J]. Angewandte Chemie International Edition, 2015, 54 :5360–5363. DOI:10.1002/anie.201501193 |
| [50] | Jiang B P, Zhou B, Shen X C, Yu Y X, Ji S C, Wen C C, Liang H. Selective probing of gaseous ammonia using red-emitting carbon dots based on an interfacial response mechanism[J]. Chemistry-A European Journal, 2015, 21 :18993–18999. DOI:10.1002/chem.201502731 |
| [51] | Ge J C, Lan M H, Zhou B J, Liu W M, Guo L, Wang H, Jia Q Y, Niu G L, Huang X, Zhou H Y, Meng X M, Wang P F, Lee C S, Zhang W J, Han X D. A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation[J]. Nature Communications, 2014, 5 :4596–4603. |
| [52] | Ge J C, Jia Q Y, Liu W M, Guo L, Liu Q Y, Lan M H, Zhang H Y, Meng X M, Wang P F. Red-emissive carbon dots for fluorescent, photoacoustic, and thermal theranostics in living mice[J]. Advanced Materials, 2015, 27 :4169–4177. DOI:10.1002/adma.v27.28 |
| [53] | Ge J C, Jia Q Y, Liu W M, Lan M H, Zhou B J, Guo L, Zhou H Y, Wang Y, Gu Y, Meng X M, Wang P F. Carbon dots with intrinsic theranostic properties for bioimaging, red-light-triggered photodynamic/photothermal simultaneous therapy in vitro and in vivo[J]. Advanced Healthcare Materials, 2016, 5 (6) :665–675. DOI:10.1002/adhm.201500720 |
| [54] | Guo L, Ge J C, Liu W M, Niu G L, Jia Q Y, Wang H, Wang P F. Tunable multicolor carbon dots prepared from well-defined polythiophene derivates and their emission mechanism[J]. Nanoscale, 2016, 8 :729–734. DOI:10.1039/C5NR07153D |